

Innovative Research
Providing state-of-the-art methodology for clinical, basic science and translational research empowers Duke Anesthesiology to explore revolutionary clinical inquiries by using innovative investigation methods.
Through significant research in neuroscience, molecular biology, molecular and human pharmacology endeavors, our team is making crucial advancements for patients worldwide.
The Quest for New Therapies to Improve Ischemic Stroke Outcome
Wei Yang, PhD, FAHA
An ischemic stroke occurs when the blood flow to part of the brain is cut off. It is the most common type of stroke and accounts for about 87% of all strokes in the nation. The high incidence of stroke-induced disability presents a major burden on families and health care systems. This burden will only increase as our population ages since age is the key non-modifiable risk factor for stroke. We currently have very few treatment options to offer stroke victims. Thus, new stroke treatment strategies are urgently needed.
Dr. Wei Yang’s long-term research goal is to develop new treatments that will improve outcome after ischemic stroke, especially in elderly patients (Figure 1). The current stroke research in his lab is focused on strategies that boost recovery of stroke-impacted brain cells and harness brain remodeling processes.
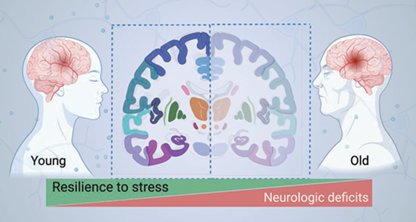
Proteins are the key biologic molecules that do most of the work in cells. Thus, protein homeostasis (proteostasis) is essential for cell survival and organismal health. Cellular proteostasis is rigorously maintained by a complex network that underpins the cell’s resilience to stress. However, this resilience declines with aging. One major component of this network resides in the endoplasmic reticulum (ER), a critical organelle for protein maturation. Disruption of ER proteostasis leads to ER stress, which activates multiple adaptive response pathways, collectively known as the unfolded protein response (UPR). The primary purpose of the UPR is to restore cellular proteostasis and thereby promote cell survival. Yang and others have demonstrated that ischemic stroke disrupts cellular proteostasis and causes ER stress in the brain. Therefore, a promising therapeutic strategy to help restore proteostasis in brain cells after stroke is to boost the UPR within the proper time window. Yang’s Molecular Neurobiology Laboratory has uncovered a wealth of insights into the role of the three UPR branches in neurons after stroke. However, little is known about the involvement of the UPR in astrocytic cells after stroke. This knowledge deficiency constitutes a major barrier to the development of UPR-based stroke therapeutics. To address this knowledge gap, his on-going research (a five-year $2,012,500 NIH R01 renewal grant) is using mouse genetic tools to manipulate individual UPR branches specifically in astrocytes and to clarify their roles in stroke. Such new knowledge will then empower the field to evaluate the therapeutic potential of targeting the UPR using various selective pharmacologic tools.
Another area of Yang’s stroke research aims to advance our understanding of the role of neuronal activity in stroke recovery. To enhance brain plasticity, neuromodulation strategies, including brain stimulation, have been explored in stroke therapy. However, clinical studies that have used various forms of brain stimulation after stroke have yielded inconsistent results, highlighting a knowledge gap about the role of neuronal activity in stroke recovery. Indeed, the underlying roles of specific neuronal populations during the stroke recovery phase are poorly understood, and it is not yet known how modulation of specific neuronal cell activity affects stroke outcome as a function of age. To address these critical questions, Yang has been closely working with his collaborators, Drs. Huaxin Sheng, Dennis A. Turner and Junjie Yao, and has designed a comprehensive, novel approach, capitalizing on DREADD-based chemogenetics, in vivo gene delivery, and advanced acoustic angiography imaging. This on-going research (a two-year $442,750 NIH R21 grant) is expected to generate critical information for the development of innovative stroke therapeutics by optimizing neuromodulation paradigms and developing new neurotechnology.
Duke Anesthesiology’s Multidisciplinary Brain Protection Program
Duke University became one of just six medical institutions in the nation to be selected as a testing laboratory site for the renowned National Institutes of Health’s (NIH) Stroke Preclinical Assessment Network (SPAN).
MISSION
The Multidisciplinary Brain Protection Program aims to discover novel and effective therapeutic strategies of brain protection and recovery through a deep mechanistic understanding of the pathophysiology that underpins brain disorders.

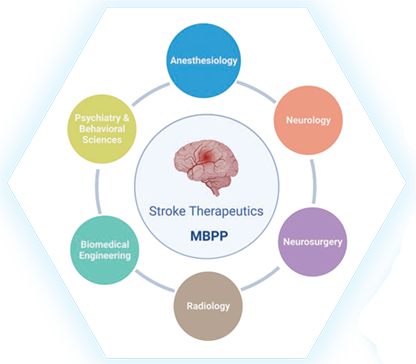
Stroke is a complex disease, demanding collaborative efforts for discovery of new, effective therapeutics. To foster team research in stroke, Yang has assumed the role as director of the Multidisciplinary Brain Protection Program (MBPP), established by Duke Anesthesiology; its investigators have expertise ranging from basic molecular biology to clinical science and span multiple departments across Duke (Figure 2). Earlier this year, Duke University became one of just six medical institutions in the nation to be selected as a testing laboratory site for the renowned National Institutes of Health’s (NIH) Stroke Preclinical Assessment Network (SPAN) to assess the efficacy of interventions selected by SPAN to improve long-term stroke outcome. The Duke SPAN site, which is funded by a three-year $1,764,172 U01 grant and led by Sheng and Yang (both of Duke Anesthesiology and the MBPP), is expected to accelerate the translation of the research pipeline and advance the discovery of promising interventions that can then be successfully advanced into clinical trials to achieve better recovery for stroke patients nationwide.
Defining the Neural Architecture of the Murine and Human Temporomandibular Joint for Advancing Orofacial Pain Therapies
Christopher Donnelly, DDS, PhD
Temporomandibular disorders (TMD) are the most common form of chronic orofacial pain, affecting five percent of adults in the United States. Despite substantial clinical and research interest in this area, progress to identify and target pathophysiological mechanisms underlying TMDs has been slow. The lack of meaningful progress is due in large part to our relatively primitive understanding of the basic neuroanatomical, molecular, and physiological features of sensory afferents present within the temporomandibular joint (TMJ) tissues.
Dr. Christopher Donnelly is the recipient of a UC2 award, titled “Neural Architecture of the Murine and Human Temporomandibular Joint,” in response to the National Institutes of Health’s Restoring Joint Health and Function to Reduce Pain (RE-JOIN) Consortium. The scope of this consortium is to acquire a comprehensive understanding of the types and distribution of nerve endings present in joint tissues to aid in the identification of molecular mechanisms that underlie chronic pain conditions involving the joints, thereby accelerating the development of new therapeutic modalities.
In this multi-PI application with collaborators from the University of Michigan, Donnelly and his team have set the ambitious goal of defining the innervation of articular and peri-articular tissues that compose joints, with an emphasis on the sensory neurons that mediate nociception. This work is significant because it will lead to an understanding of the neurological mechanisms that underlie chronic orofacial pain in patients with temporomandibular disorders and other orofacial pain conditions. With a background in dentistry, Donnelly not only brings innovation to this project, but also relevant clinical expertise in the field.
“This research will comprehensively define the cellular, molecular and neurophysiological properties of the sensory neurons that innervate tissues of the temporomandibular joint. Beyond accelerating our understanding of orofacial somatosensation, this research will reveal mechanisms of joint pain that can be used to develop new clinical interventions and pain therapies.” – Dr. Christopher Donnelly
The project focuses on elucidating the anatomy of the temporomandibular joint due to the severe health burden of temporomandibular disorders, one the most common orofacial pain conditions. The team’s overarching objective is to create a comprehensive atlas of the sensory neurons that innervate the tissues which compose the temporomandibular joint in mice and in humans. This anatomical atlas with physiological and functional mapping will be developed both in health and pathological states.
To accomplish their goals, the team proposes to develop and implement several innovative new tools and techniques to study the TMJ, including magnetic resonance imaging with stereotactic injections of TMJ tissues, fluorescent labeling of afferents fibers with transcriptomic analyses reflective of innervation plasticity. They will also use advanced techniques to map somatosensory neuron subpopulations innervating the TMJ. One of the highly progressive and innovative aspects of this research that brings a very comprehensive characterization of the structure, function and dysfunction of the TMJ relies on the in vivo recording of sensory neuron activity during orofacial function and in the presence of acute or chronic pain stimuli, with concurrent behavioral phenotyping and chemogenetic modulation.
The research experiments are expected to produce synergistic information describing the neuroanatomical, molecular, and functional properties of TMJ-innervating sensory neurons in mice and humans, culminating in the creation of a publicly accessible platform to facilitate the conceptual understanding of TMJ sensory function in health and chronic pain states. While this project is focused on the TMJ, the innovative tools and techniques developed in this research will be useful as a template for future studies of other joints.
AWARD: Three-year $5,734,530 UC2 grant
Funding Source: The National Institutes of Health’s (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
HIGHLIGHT: Selected as one of just five UC2 awards that make up the RE-JOIN Consortium – part of the broader NIH Helping to End Addiction Long-term (HEAL) Initiative, an aggressive trans-agency effort to accelerate scientific solutions to treat pain and end the national opioid epidemic.
Neuro-Immune Interactions in Postoperative Delirium
Niccolò Terrando, BSc (hons), DIC, PhD
Delirium is a quintessential neurologic complication characterized by changes in cognition, attention and arousal. Aging is the most prominent risk factor for postoperative delirium; however, it remains unclear how aging contributes to the pathogenesis of this common neurologic complication. Delirium is observed frequently after routine surgical procedures such as orthopedic surgery, where it greatly impacts patients, families and caregivers, and adds significant costs to our health care. Delirium is particularly insidious in vulnerable patients, especially those with pre-existing neurodegeneration such as Alzheimer’s disease (AD). More than five million people in the United States suffer from dementia, including AD, and commonly require surgical interventions, such as orthopedic surgery. After a common fracture repair, postoperative delirium occurs in up to 89% of patients with preexisting dementia. Delirium in these vulnerable subjects further associates with poorer prognosis and even two-fold greater risk for one-year mortality compared to patients without dementia or delirium. Despite its prevalence, the mechanisms underlying this complication remain unknown and without effective therapies.
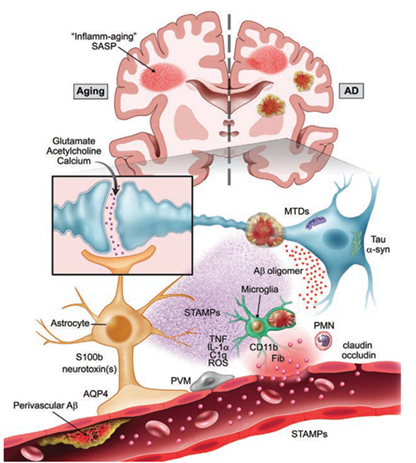
Dr. Niccolò Terrando and his team of investigators are dedicated to understanding how surgery drives delirium in aged and vulnerable individuals. Using an integrated multidisciplinary and translational approach, they are particularly interested in how the activation of the immune system drives brain inflammation (i.e. neuroinflammation), impairing neuronal function and leading to acute changes in behavior during aging and neurodegeneration (Figure 1). Their long-term goal is to define the mechanisms underlying delirium and to develop effective approaches to reduce this devastating complication. Over the years, these efforts have been supported by multiple grants from the National Institute on Aging. And, through the recent renewal of a five-year $4,311,630 R01 grant, they continue to advance new knowledge on how surgery impacts brain function in AD-like mice by focusing on specific neuro-immune signaling at the blood-brain barrier. Previous work from Terrando and his team has discovered that orthopedic surgery in mice triggers acute changes in beta-amyloid, a key hallmark of the AD brain, and is accompanied by marked changes in the brain vasculature. The blood-brain barrier is a key interface responsible for protecting and regulating the brain microenvironment from peripheral signals and is gaining significant attention in postoperative delirium. To better interrogate the immune interactions at the blood-brain barrier, Terrando and his team have developed new tools leveraging organ-on-chip technology (Figure 2) combined with animal models that have uncovered changes in new cellular targets, including pericytes and perivascular macrophages, that become dysfunctional after surgery. They are currently applying these tools to model more complex interactions in the AD-vulnerable brain and better reflect the brain state during delirium superimposed on dementia.
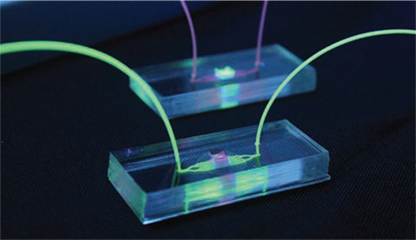
Over the duration of this project, multiple collaborations have generated new paradigms to 1) better characterize delirium-like behavioral states with William Wetsel and Ramona Rodriguiz at the Duke Behavioral and Endocrine Core, 2) develop novel bioelectronic approaches to safely regulate the immune response after surgery harnessing the vagus nerve with Warren Grill at Duke Biomedical Engineering (BME), 3) implement new microphysiological systems to model neuroimmune interactions with Shyni Varghese at Duke BME and the Harris Gelbard and James McGrath laboratories at the University of Rochester Medical Center, and 4) apply innovative analytical tools to define the single-cell landscape in response to surgical trauma and delirium with Katerina Akassoglou at the Gladstone Institutes. These efforts are contributing to an advanced understanding of how surgery affects the brain and are already resulting in translational studies that support the premise that similar pathological features are also present in the human brain after anesthesia and surgery.
Over a decade ago, in collaboration with the Akassoglou laboratory, Terrando and research teams discovered that fibrinogen, a critical driver of neuroinflammation in multiple neurologic conditions, is deposited in the brain after orthopedic surgery. In this R01 renewal, these teams will continue to define how surgery impairs the blood-brain barrier, both using animal models as well as organ-on-chip technology to dissect neuro-immune communication in AD. The rationale for the proposed research is that successful completion will advance our understanding of how surgery affects the immune system and will provide new tools of relevance to delirium, neurodegeneration and aging. Such knowledge is highly significant because it has the potential to explain how surgery impacts the vulnerable brain and identify novel targets to prevent postoperative delirium.
MTDs: Mitochondrial damage associated molecular patterns | PMN: Polymorphonuclear Leukocytes | ROS: Reactive Oxygen Species | PVM: Perivascular Macrophage
PUBLICATION REFERENCES:
- Wang P, Velagapudi R, Kong C, Rodriguiz R, Wetsel W, Yang T, Berger M, Gelbard HA, Colton C, Terrando N, Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia, Alzheimers Dement, 16(5):734-749, 2020. PMID: 32291962.
- Yang T, Velagapudi R, Kong C, Ko U, Kumar V, Brown P, Franklin NO, Zhang X, Caceres AI, Min H, Filiano AJ, Rodriguiz RM, Wetsel WC, Varghese S, Terrando N. Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice. Br J Anaesth. 2023;130(2):e370-e380. PMID: 35778276.
