
Jorn A. Karhausen, MD – 2012
2012 Final DIG Report | "Determinants of Intestinal Epithelial Wound Healing"
The Study
This study is aimed at unraveling compensatory mechanisms that help the intestinal mucosa respond to injury due to ischemia (interruption of blood flow) and reperfusion (restoration of blood supply). In cardiac surgery, pronounced abnormalities of blood flow are observed particularly in the small blood vessels within the intestinal wall. In the past, it has been repeatedly hypothesized that the resulting lack of tissue oxygenation leads to breaches of intestinal barrier integrity, bacterial translocation, activation of gut resident inflammatory cells, and consequently, to systemic inflammatory responses. Based on my previous work, I had hypothesized that the intestinal epithelium is not only uniquely susceptible to such ischemic injury, but also uniquely equipped to respond to it.
Findings
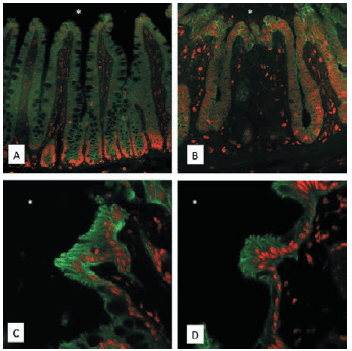
In a mouse model, our research team observed that the intestinal lining was profoundly injured immediately after ischemia, but that within three to five hours after reperfusion, epithelial cells had spread out to fully cover the wound. This swift recovery of epithelial integrity did not seem to be immediately dependent on the regulatory cycle identified in our initial proposal, consisting of the zinc finger E-box binding homeobox factors 1 and 2 (ZEB-1, -2) and the miR-200 family of micro RNAs. Extensive research based on protein and RNA profiles did not reveal significant changes in these components, and an attempt to recapitulate these conditions under cell culture conditions using genetically modified cell lines showed no functional differences to unmodified (wild-type) cells.
As we investigated possible explanations and alternatives, we observed a significant increase of protein SUMOylation in the intestinal epithelium following ischemia-reperfusion (fig. 1). SUMO, or Small Ubiquitin-like Modifiers, are small proteins that alter the function of other cellular proteins by direct binding, and thus modifying their function, cellular localization, or stability. Changes in SUMOylation have been observed in an ischemia-reperfusion setting and are thought to predominantly contribute to tissue-protective anti-inflammatory responses.
Next Steps
As a consequence of these results, I have begun collaborating with Wulf Paschen, PhD, and Wei Yang, PhD, of Duke University. Both are experts in research on SUMO-modifications in ischemia, and over the last few years have developed innovative tools to study these modifications in cell culture and animal models. With their support, I have generated SUMO-deficient cell lines and am characterizing the pattern of SUMOylation in the context of intestinal ischemia-reperfusion injury. Preliminary data gathered through this work has served to apply for both internal (Duke Chancellor`s Development Fund) and external (American Gastroenterological Association, Pilot Grant) pilot funding. Important aspects of these results have also been incorporated in a K08-Mentored Clinical Scientist Career Development Award application, which I have just submitted to the National Institutes of Health. The DIG has funded important observations central to my research endeavors, and I expect significant returns of this investment through the initiated projects.