Lab Description
The long-term goal of research in the Molecular Neurobiology Laboratory is to develop novel and effective therapeutic strategies to improve outcomes after ischemic stroke and cardiac arrest. We investigate various pathophysiologic aspects of ischemia/reperfusion injury. Critically, our research takes into account that age is a key risk factor for ischemic stroke and cardiac arrest. We not only aim to identify molecular and cellular mechanisms that underpin ischemia/reperfusion-induced tissue damage, especially in the brain, after ischemic stroke or cardiac arrest, but also explore the translational potential of our key findings. The lab uses a wide range of approaches, including molecular, cellular, omics, and viral techniques, electrophysiology, immunologic analyses, genetic mouse models, animal models of human disease, and behavioral testing.
Major Research Interests
Ischemic Stroke
Every year, nearly 800,000 people in the United States experience a stroke, and most of these are ischemic strokes – a leading cause of death, as well as long-term disability, that present a major burden for families and health care systems. During the acute phase, ischemic stroke rapidly initiates many pathologic processes that lead to brain cell death, while during the recovery phase, endogenous neurorestorative processes that contribute to spontaneous recovery of neurologic function are believed to be activated in the post-stroke brain. This 2-phase theory largely guides the current search for therapeutic strategies to improve stroke outcome, ie, neuroprotective strategies during the acute phase, and neurorestorative strategies during the recovery phase. Our group is currently studying endogenous protective processes/pathways in both post-stroke phases. These include proteostasis-related pathways such as the unfolded protein response (UPR), SUMOylation, and O-GlcNAcylation, and modulation of neuronal activity.
Cardiac Arrest and Resuscitation
Many advances in resuscitation have led to increased numbers of cardiac arrest (CA) patients who survive the initial arrest event and are admitted to the Intensive Care Unit. Unfortunately, among this growing patient population, mortality and morbidity remain strikingly high. This is attributed to a complex set of pathophysiologic processes that include the ischemia/reperfusion-induced cascade of pathologic events in various organs and systemic immune response. Therefore, our CA research is focused not only on individual organs such as the brain, lung and intestine, but also on the systemic hormonal and immune responses.
Lab Members
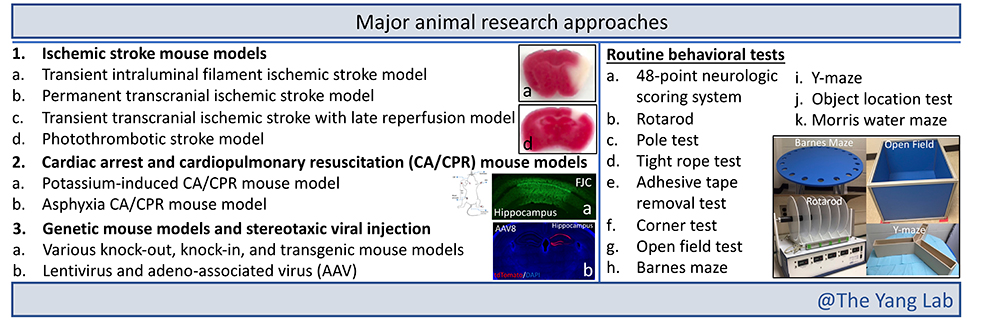
Research Approaches
Selected Publications
- Wang W, Li R, Miao W, Evans C, Lu L, Lyu J, Li X, Warner DS, Zhong X, Hoffmann U, Sheng H, Yang W. (2021) Development and Evaluation of a Novel Mouse Model of Asphyxial Cardiac Arrest Revealed Severely Impaired Lymphopoiesis After Resuscitation. J Am Heart Assoc. 10:e019142.
- Wang Z, Li X, Spasojevic I, Lu L, Shen Y, Qu X, Hoffmann U, Warner DS, Paschen W, Sheng H, Yang W. (2021) Increasing O-GlcNAcylation is neuroprotective in young and aged brains after ischemic stroke. Exp Neurol. 339:113646.
- Shen Y, Li R, Yu S, Zhao Q, Wang Z, Sheng H, Yang W. (2021) Activation of the Activating Transcription Factor 6 Signaling Pathway in Neurons Improves Outcome After Cardiac Arrest in Mice. J Am Heart Assoc. 10:e020216.
- Zhao Q, Shen Y, Li R, Wu J, Lyu J, Jiang M, Lu L, Zhu M, Wang W, Wang Z, Liu Q, Hoffmann U, Karhausen J, Sheng H, Zhang W, Yang W. (2021) Cardiac arrest and resuscitation activates the hypothalamic-pituitary-adrenal axis and results in severe immunosuppression. J Cereb Blood Flow Metab, 41(5):1091-1102.
- Turner DA, Yang W. (2021) Phase-specific manipulation of neuronal activity: a promising stroke therapy approach. Neural Regen Res. 16(7):1425-1426.
- Li R, Shen Y, Li X, Lu L, Wang Z, Sheng H, Hoffmann U, Yang W. (2021) Activation of the XBP1s/O-GlcNAcylation Pathway Improves Functional Outcome after Cardiac Arrest and Resuscitation in Young and Aged Mice. Shock DOI: 10.1097/shk.0000000000001732.
- Karhausen J, Ulloa L, Yang W. (2021) SUMOylation Connects Cell Stress Responses and Inflammatory Control: Lessons from the Gut as a Model Organ. Front Immunol. 12:646633.
- Zhang D, Li R, Chen M, Vu T, Sheng H, Yang W, Hoffmann U, Luo J, Yao J. (2021) Photoacoustic imaging of in vivo hemodynamic responses to sodium nitroprusside. J Biophotonics 26;e202000478
- Shemetov AA, Monakhov MV, Zhang Q, Canton-Josh JE, Kumar M, Chen M, Matlashov ME, Li X, Yang W, Nie L, Shcherbakova DM, Kozorovitskiy Y, Yao J, Ji N, Verkhusha VV. (2021) A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat Biotechnol. 39:368–377
- Jiang M, Li R, Lyu J, Li X, Wang W, Wang Z, Sheng H, Zhang W, Karhausen J, Yang W. (2020) MCC950, a selective NLRP3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation. J Neuroinflammation. 17: 256.
- Wang YC, Li X, Shen Y, Lyu J, Sheng H, Paschen W, Yang W. (2020) PERK (Protein Kinase RNA-Like ER Kinase) branch of the unfolded protein response confers neuroprotection in ischemic stroke by suppressing protein synthesis. 51(5):1570-1577.
- Wang YC, Galeffi F, Wang W, Li X, Lu L, Sheng H, Hoffmann U, Turner DA, Yang W. (2020) Chemogenetics-mediated acute inhibition of excitatory neuronal activity improves stroke outcome. Exp Neurol. 326:113206.
- Yu S, Galeffi F, Rodriguiz RM, Lyu J, Wang Z, Shen Y, Ran Li, Bernstock JD, Johnson KR, Liu S, Sheng H, Turner DA, Wetsel WC, Paschen W, Yang W. (2020) Small ubiquitin-like modifier 2 (SUMO2) is critical for memory processes in mice. FASEB J. 34(11):14750-14767.
- Li W, Chopp M, Zacharek A, Yang W, Chen Z, Landschoot-Ward J, Venkat P, Chen J. (2020) SUMO1 Deficiency Exacerbates Neurological and Cardiac Dysfunction after Intracerebral Hemorrhage in Aged Mice. Transl Stroke Res. DOI: 10.1007/s12975-020-00837-6
- Yu HI, Hsu T, Maruyama EO, Paschen W, Yang W, Hsu W. (2020) The requirement of SUMO2/3 for SENP2 mediated extraembryonic and embryonic development. Dev Dyn. 249(2):237-244.
- Bernstock JD, Ye DG, Estevez D, Chagoya G, Wang YC, Gessler F, Hallenbeck JM, Yang W. (2020) The Role of SUMOylation and Ubiquitination in Brain Ischaemia: Critical Concepts and Clinical Implications. Curr Issues Mol Biol. 35:127-144.
- Ma S, Chu D, Li L, Creed JA, Ryang YM, Sheng H, Yang W, Warner DS, Turner DA, Hoffmann U. (2019) Argon inhalation for 24 hours after onset of permanent focal cerebral ischemia in rats provides neuroprotection and improves neurologic outcome. Crit Care Med. 47(8):e693-e699.
- Wang Z, Yang W. (2019) Impaired capacity to restore proteostasis in the aged brain after ischemia: Implications for translational brain ischemia research. Neurochem Int. 127:87-93.
- Shen Y, Yan B, Zhao Q, Wang Z, Wu J, Ren J, Wang W, Yu S, Sheng H, Crowley S, Ding F, Paschen W, Yang W. (2018) Aging is associated with impaired activation of protein homeostasis-related pathways after cardiac arrest in mice. J Am Heart Assoc. 7: e009634.
- Lan B, Liu W, Wang YC, Shi J, Li Y, Xu S, Sheng H, Zhou Q, Zou J, Hoffmann U, Yang W, Yao J. (2018) High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed Opt Express. 9(10):4689-4701.
- Karhausen J, Bernstock J, Johnson K, Sheng H, Yang W, Hallenbeck J, Paschen W. (2018) Ubc9 overexpression and SUMO1 deficiency independently protect from intestinal ischemia/reperfusion injury. Lab Invest. 98(6):799-813.
- Bernstock JD, Ye D, Smith JA, Lee Y, Gessler FA, Yasgar A, Kouznetsova J, Jadhav A, Wang Z, Pluchino S, Zheng W, Simeonov A, Hallenbeck JM, Yang W. (2018) Quantitative high-throughput screening identifies cytoprotective molecules that enhance SUMO-conjugation via the inhibition of SUMO-specific protease 2 (SENP2). FASEB J. 32(3):1677-1691.
- Bernstock JD, Yang W, Ye DG, Shen Y, Pluchino S, Lee Y, Hallenbeck JM, Paschen W. (2018) SUMOylation in Brain Ischemia: Patterns, Targets, and Translational Implications. J Cereb Blood Flow Metab. 38(1):5-16.
- Liu H, Yu Z, Li Y, Xu B, Yan B, Paschen W, Warner DS, Yang W, Sheng H. (2018) Novel Modification of Potassium Chloride Induced Cardiac Arrest Model for Aged Mice. Aging Dis. 9(1):31-39.
- Bernstock J, Ye D, Gessler F, Lee YJ, Peruzzotti-Jametti L, Baumgarten P, Johnson K, Maric D, Yang W, Koegel D, Pluchino S, Hallenbeck (2017) Topotecan is a potent inhibitor of SUMOylation in glioblastoma multiforme and alters both cellular replication and metabolic programming. Sci Rep. 7(1):7425.
- Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S, Warner DS, Paschen W, Yang W. (2017) XBP1-dependent O-GlcNAcylation is neuroprotective in ischemic stroke in young mice and its impairment in aged mice is rescued by thiamet-G. Stroke. 48(6):1646-1654.
- Zhang L, Liu X, Sheng H, Liu S, Li Y, Zhao JQ, Warner DS, Paschen W, Yang W. (2017) Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain. Neuroscience. 20 (343):190-212.
- Yu Z, Sheng H, Liu S, Zhao S, Glembotski CC, Warner DS, Paschen W, Yang W. (2017) Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J Cereb Blood Flow Metab. 37(3):1069-1079.
- Yang W, Paschen W. (2016) Unfolded protein response in brain ischemia: A timely update. J Cereb Blood Flow Metab. 36(12):2044–2050.
- Yang W, Sheng H, Wang H. (2016) Targeting the SUMO pathway for neuroprotection in brain ischemia. Stroke Vasc Neurol.1(3):101-107.
- Liu S, Sheng H, Yu Z, Paschen W, Yang W. (2016) O-linked β-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: Implications for impaired functional recovery from ischemic stress. J Cereb Blood Flow Metab. 36(2):393-8.
- Yang W, Paschen W. (2015) SUMO proteomics to decipher the SUMO-modified proteome regulated by various diseases. Proteomics. 15(5-6):1181-91.
- Wang L, Wansleeben C, Zhao S, Miao P, Paschen W, Yang W. (2014) SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep. 15(8):878-85.
- Yang W, Sheng H, Thompson JW, Zhao S, Wang L, Miao P, Liu X, Moseley MA, Paschen W. (2014) Small Ubiquitin-Like Modifier 3-Modified Proteome Regulated by Brain Ischemia in Novel Small Ubiquitin-Like Modifier Transgenic Mice: Putative Protective Proteins/Pathways. Stroke 45(4):1115-22.
- Iwabuchi M, Sheng H, Thompson JW, Wang L, Dubois LG, Gooden D, Moseley M, Paschen W, Yang W. (2014) Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. J Cereb Blood Flow Metab. 34(3):425-32.
- Wang L, Rodriguiz RM, Wetsel WC, Sheng H, Zhao S, Liu X, Paschen W, Yang W. (2014) Neuron-specific SUMO1-3 knockdown in mice impairs episodic and fear memories. J Psychiatr Neurosci. 39(2):130148.
- Yang W, Wang L, Paschen W. (2013) Development of a high-throughput screening assay for inhibitors of small ubiquitin-like modifier proteases. Biomol. Screen. 18(5):621-8.
- Yang W, Wang L, Roehn G, Pearlstein RD, Ali-Osman F, Pan H, Goldbrunner R, Krantz M, Harms C, Paschen W. (2013) SUMO1-3 is activated in human astrocytic brain tumors and is required for glioblastoma cell survival. Cancer Sci. 104(1):70-7.
- Wang Z, Wang R, Sheng H, Sheng SP, Paschen W, Yang W. (2013) Transient ischemia induces massive nuclear accumulation of SUMO2/3-conjugated proteins in spinal cord neurons. Spinal Cord. 51(2):139-43.
- Wang L, Ma Q, Yang W, Mackensen GB, Paschen W. (2012) Moderate hypothermia induces marked increase in levels and nuclear accumulation of SUMO2/3-conjugated proteins in neurons. Neurochem. 123, 349-59.
- Yang W, Thompson JW, Wang Z, Wang L, Sheng H, Foster MW, Moseley MA, Paschen W. (2012) Analysis of Oxygen/Glucose-Deprivation-Induced Changes in SUMO3 Conjugation Using SILAC-Based Quantitative Proteomics. Proteome Res. 11, 1108-17.
- Datwyler AL, Lättig-Tünnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. (2011) SUMO2/3 conjugation is an endogenous neuroprotective mechanism. Cereb. Blood Flow Metab. 31, 2152-59.
- Wang Z, Yang W, Britz GW, Lombard FW, Warner DS, Sheng H. (2010) Development of a simplified spinal cord ischemia model in mice. J Neurosci Methods.189, 246-51.
- Yang W, Ma Q, Mackensen G, Paschen W. (2009) Deep hypothermia markedly activates the small ubiquitin-like modifier (SUMO) conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. J. Cereb. Blood Flow Metab. 29, 886-90.
- Yang W and Paschen W. (2009) The endoplasmic reticulum and neurological diseases. Neurol.219(2):376-81.
- Sheng H, Yang W, Fukuda S, Tse HM, Paschen W, Johnson K, Batinic-Haberle I, Crapo JD, Pearlstein RD, Piganelli J, Warner DS. (2009) Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radic. Biol. Med. 47(7): 917-23.
- Yang W, Sheng H, Homi M, Warner DS, and Paschen W. (2008) Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation – a new target for therapeutic intervention? Neurochem. 106, 989-999
- Yang W, Sheng H, Warner DS, and Paschen W. (2008) Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier (SUMO) conjugation. Cereb. Blood Flow Metab. 28, 892-896.
- Yang W, Sheng H, Warner DS, and Paschen W. (2008) Transient global cerebral ischemia induces a massive increase in protein sumoylation. Cereb. Blood Flow Metab. 28, 269-79.
- Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M, Paschen W, Piccirella S, Senesi S, Sitia R, Wang M, Yang W. (2007) Endoplasmic reticulum stress. Ann N Y Acad Sci. 1113:58-71.






