
Innovative Research
Providing state-of-the-art methodology for clinical, basic science and translational research empowers Duke Anesthesiology to explore revolutionary clinical inquiries by using innovative investigation methods.
Through significant research in neuroscience, molecular biology, molecular and human pharmacology endeavors, our team is making crucial advancements for patients worldwide.
Targeted Treatment for Lung Diseases
By Satya Achanta, DVM, PhD, DABT

Satya Achanta, DVM, PhD, DABT, director of the Medical Countermeasures and Pain Translational Laboratory, has been pursuing medical countermeasures research for more than 10 years. The Achanta lab focuses on identifying drug targets and developing mechanism-based targeted medical countermeasures against pulmonary diseases.
Phosgene Gas: A Multidimensional Chemical Threat Agent With No Effective Treatment
Phosgene (carbonyl chloride, COCl2; military designation, CG) is a highly toxic chemical that exists as a gas at room temperature. The toxic effects of phosgene gas were first reported in 1899 by a group of surgeons and anesthesiologists when chloroform was converted into phosgene. In the modern era, phosgene is widely used as an intermediate in the chemical manufacture of pharmaceuticals, polymers, dyes, and other products. Despite its use as a chemical weapon since World War I, there is no effective antidote against phosgene inhalation-induced lung disease. Therefore, phosgene gas remains an important threat, potentially released in industrial accidents, or diverted or synthesized by terrorist groups.
Following a latency period of about 6-8 hours after inhalation, the pulmonary effects of phosgene gas manifest. These include shortness of breath, cough and severe pulmonary edema leading to high mortality. Chronic effects in survivors include persistent inflammation, pulmonary remodeling, emphysema, and fibrosis. Currently, there are no mechanism-based treatment options for phosgene gas-induced lung injuries. Symptomatic treatment is considered standard of care, with limited success. Only a few experimental therapeutics are in pre-clinical testing and additional candidate strategies are required. Repurposing current United States Food and Drug Administration (US FDA)-approved drugs as potential medical countermeasures has been encouraged by the NIH CounterACT program that funds research studies in developing medical countermeasures.
As clinical trials are not feasible and ethical, the US FDA has a provision to approve drugs based on data from animal models under the animal rule for chemical threat agents. Therefore, the first challenge in this research program is to develop reproducible animal models that recapitulate the natural history of disease progression in humans. As a research veterinarian, Achanta is well-known for developing rodent and non-rodent animal models in this research field.
Successful completion of this research could mark a pivotal advancement in targeted treatments against phosgene gas injuries, addressing a significant gap in current medical countermeasures. Achanta and his team are committed to paving the way in conducting studies that support the Biomedical Research Development Authority and potentially expediting FDA approval under the Animal Rule, ultimately offering hope for those vulnerable to this deadly chemical.
Novel Approaches
The National Institutes of Health’s National Institute of Environmental Health Sciences (NIEHS) has awarded Achanta, a two-year UG3 grant for his project titled, “Identification and Optimization of Medical Countermeasures for Phosgene Inhalation Injuries.” In this initial phase (UG3), Achanta will evaluate the therapeutic efficacy of two classes of drugs, including FDA-approved drugs and late-stage novel clinical drug candidates as potential medical countermeasures for phosgene inhalation injuries.
The renin-angiotensin-aldosterone system (RAAS) plays a key role in cardiopulmonary homeostasis. However, RAAS is dysregulated during acute respiratory distress syndrome (ARDS), contributing to underlying pathophysiology. Achanta has found that angiotensin-converting enzyme (ACE) levels have significantly increased in phosgene-exposed mice compared to air-exposed animals. As a first approach, Achanta and his team will evaluate various FDA-approved and investigational RAAS modulators (Figure 1).
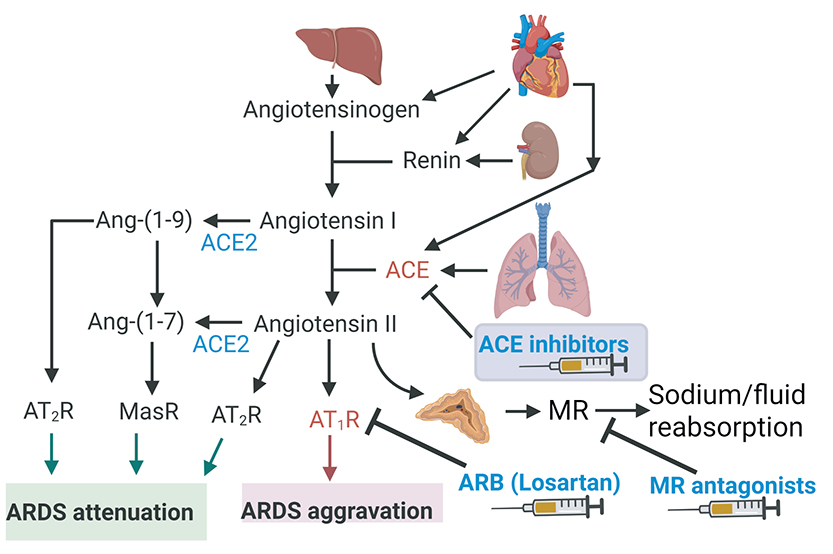
In the second approach, Achanta has been focusing on stimulating pro-resolving pathways to accelerate the resolution phase of inflammation. Some of the pro-resolving mediators that are generated during the inflammation cascade are short-lived due to degradation by an enzyme called soluble epoxide hydrolase (sEH). Achanta has found that sEH enzyme levels have significantly increased after phosgene inhalation injury and small molecule inhibitors of sEH improved survival rates and mitigated inflammation in the pilot data.
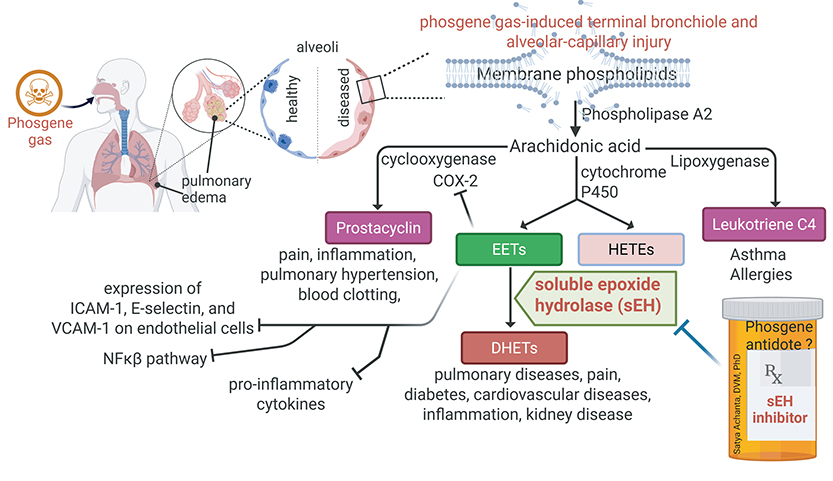
In the UG3 phase, Achanta and his team will evaluate short-term and long-term therapeutic effects of potential RAAS modulators and sEHIs in rodent models. Upon successful completion of milestones in the UG3 phase, a three-year UH3 phase of funding will be granted to evaluate the most effective therapeutic drugs in non-rodent models — bringing the total funding for the five-year project to $3.25 million. BP
Funding Sources:
Achanta received funding (R21, R01, R56, UG3/UH3) from the NIH CounterACT program to develop animal models, understand pathophysiology, identify drug targets, and discover forensic diagnostic biomarkers and potential medical countermeasures
Listening to the Brain—and the Patient: Using Attentional Neuroscience to Rewire Perioperative Cognition
By Leah Acker, MD, PhD

Poor cognitive control is a familiar comic trope. Whether it is Homer Simpson’s helplessness around donuts, Michael Scott’s fixation on a joke that turns wildly inappropriate, or the infamous Cookie Monster, we laugh because we recognize the discomfort of losing control over our thoughts and actions. In real life, though, cognitive control—especially the ability to focus one’s attention—is serious business.
Even in ideal circumstances, the brain does not have enough bandwidth to attend to everything in our environment. Attentional control—a key form of cognitive control—helps us to overcome this limitation by allocating neural resources to the most relevant environmental stimuli. Attentional control helps us follow a conversation in a noisy café or stay focused during a meeting, despite a pencil-tapping colleague. More broadly, it allows us to make the most of limited neural resources to support goal-directed behavior. When those resources are diminished—as they often are with aging, illness, or injury—attentional control becomes even more essential.
In the Anesthesiology Cognitive Neuroscience and Engineering Research (ACkER) Lab, we study attentional resilience—the ability to retain strong attentional control despite physiological stress. We hypothesize that attentional resilience protects some older adults from developing postoperative delirium, an acute confusional state that affects up to half of older surgical patients. Delirium increases the risk of later dementia and death, yet no pharmacological treatment exists, in part because delirium is not caused by a single factor. Instead, it likely reflects a final common pathway: a combination of physiological stressors—like inflammation or metabolic abnormalities—that drain neural resources beyond the point where attentional control can compensate.
To explore the mechanisms that support attentional resilience, we are enrolling 150 older adults in a new NIH-funded study, Cognitive Health, Attentional Resilience, and Effects on Delirium (CHARMED). Before surgery, participants complete attention-based tasks while we record their electrical brain activity using electroencephalography (EEG). EEG captures real-time responses to stimuli and brain rhythms such as alpha (7–13 Hz) oscillations, which reflect overall attentional state. Participants also undergo advanced neuroimaging, including functional MRI and diffusion imaging, to map the structure and connectivity of attention-related brain networks. Overall, we aim to understand what makes some brains more resilient, identify patients at highest risk for delirium, and develop interventions that enhance attentional control.
Preliminary findings from our group suggest that even simple EEG-based measures may offer useful insights. For example, we found that patients whose alpha oscillations failed to attenuate when shifting from eyes-closed to eyes-open were more likely to experience attentional problems after surgery. This brief test (published in the British Journal of Anaesthesia: doi.org/10.1016/j.bja.2023.10.037), which takes just a few minutes and uses existing EEG monitors, could one day serve as a low-cost, preoperative screening tool.
Attention is shaped not only by the brain, but also by the body—particularly through the autonomic nervous system and what we call the brain-heart-immune axis (BHI-A). In a recent NIH-funded study—Heart Rate Variability in Postoperative Delirium and Postoperative Inflammatory Endpoints (HiPPIE)—we used at-home wearable devices to measure heart rate variability before surgery, a marker of BHI-A function. We found that diminished autonomic control was linked to a higher risk of postoperative delirium, reflecting something many of us have experienced: trouble concentrating when we are emotionally stressed or physically tense.
“This research will advance our understanding of why some older adults maintain strong attentional performance post-surgery while others do not.”
– Dr. Leah Acker
Research Discovery Timeline:
2022
- ACkER Lab founded
- HiPPIE clinical study launched “Heart Rate Variability in Postoperative Delirium and Postoperative Inflammatory Endpoints”
- 2-year $322,000 NIH R03: The Role of the Aging Brain-Heart-Immune Axis in Postoperative Delirium”
- SNACC Bill Young Research Award
- 2-year Duke Pepper Center: Research Education Core Grant
2023
- POTENT clinical study launched “Pre-Op taVNS Effects on Neuro-Cognitive and Neuro-Inflammatory Trends”
- 2-year FAER GEMSSTAR Award
- 3-year Duke Health Strong Start Award
- Duke Anesthesiology Dream Innovation Grant
2024
- HiPPIE and POTENT clinical studies completed
- CHARMED study launched “Cognitive Health, Attentional Resilience, and Effects on Delirium”
- 5-year $3.82M NIH R01: “Attentional Resilience in Older Adults”
In a parallel pilot study, partly funded by a Duke Anesthesiology DREAM Innovation Grant, we tested transcutaneous auricular vagus nerve stimulation (taVNS), a noninvasive method that gently stimulates the vagus nerve through the ear. First, we demonstrated that taVNS was safe, well tolerated, and feasible for at-home use in older surgery patients. Then, in a follow-up study of 30 healthy college students performing attentional tasks, we found that taVNS altered EEG responses compared to sham stimulation—suggesting a possible role in enhancing attentional control. We look forward to larger studies testing taVNS as a potential intervention to support attentional resilience.
Finally, my team and I listen carefully to patients themselves. In a recent secondary analysis of our HiPPIE cohort, we analyzed both survey data and patients’ own words to understand their experiences in the days before surgery. These data revealed an “overwhelmed” phenotype—patients who described too many moving parts and too little control. The “overwhelmed” group faced worse outcomes, including more pain, longer hospital stays, and higher delirium rates. The patient experiences we observed may reflect more than emotional distress; they may reveal early vulnerabilities in cognitive control. In future work, we plan to link the overwhelmed phenotype to neural measures of attention, creating tools that bridge patient experience and brain-based risk markers.
While poor cognitive control is a familiar TV joke, even Cookie Monster eventually gained enough attentional control to forgo cookies and embrace vegetables. Here in the real world, we aim to help patients do the same—strengthening attentional control through the vulnerable perioperative period to promote neurocognitive resilience, ultimately supporting better health and greater patient autonomy. BP
Uncovering the Pathology of Oxidative Tissue Injury
By Heath Gasier, PhD

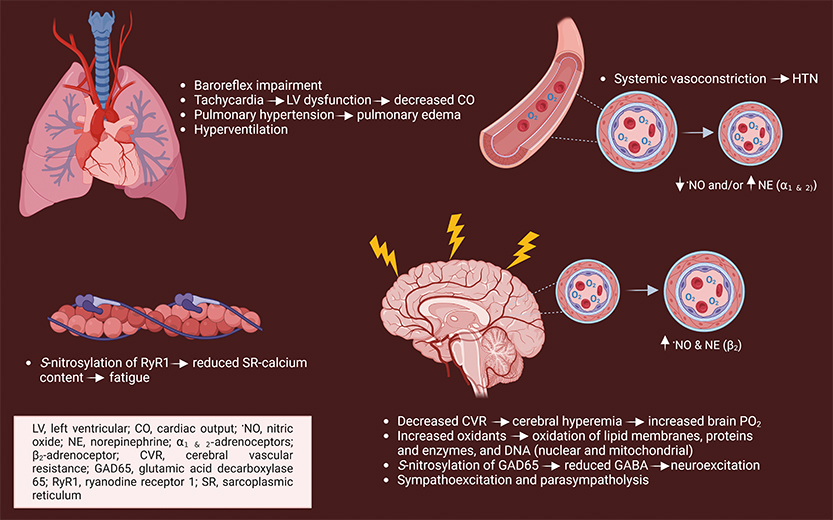
When I was first introduced to the field in 2009, “why” oxygen is toxic to mammals seemed straightforward. The PO2 within cells is contained within a narrow range (~2 - 6%) — above this will exceed mitochondrial respiratory demand in most instances and antioxidant defenses, leading to increased oxidant production. Since antioxidant capacities are exceeded, oxidation of lipids, proteins and nucleic acids ensues, resulting in lung injury and possibly tonic-clonic seizures and death. Precisely “how” this occurs is unknown, a question I was encouraged to study out of “necessity” for the US Navy. Specifically, if you breathe pure oxygen, decompression sickness is no longer a concern. For two years, I studied under Dr. Claude Piantadosi and Dr. Ivan Demchenko at the Duke Center for Hyperbaric Medicine and Environmental Physiology (CHMEP). Our investigations identified or expanded upon existing knowledge of the physiological responses in hyperbaric oxygen (HBO2) (Figure 1) and led to pharmacological testing. The goal? To dive deeper for extended periods, critical for the preservation of undersea superiority.
After retiring from the US Navy in 2019, Piantadosi and Dr. Richard Moon invited me to return to the CHMEP and continue researching the mechanisms of oxygen toxicity. Despite my enthusiasm, I realized the hypotheses and aims that should be tested required instrumentation not available within the CHMEP’s Oxygen Transport Laboratory. For instance, the Office of Naval Research (ONR) supported research ($228K) directed at determining whether HBO2-induced fatigue during aerobic exercise reported in US Navy divers was due to impaired mitochondrial activity or dysregulated calcium trafficking. Specifically, rodent metabolic treadmill and respirometry systems were required to confirm that repeated HBO2 caused fatigue in mice like humans and if it is due to a lower mitochondrial bioenergetic capacity. Solution? Submission of proposals to the Defense University Research Instrumentation Program (DURIP), designed to enhance the capabilities of Department of Defense directed research at US institutions. In 2021 and 2022, I received DURIP awards ($191K) to procure a Columbus Instruments Oxymax Metabolic Treadmill for mice and rats along with an Agilent Seahorse XF HS Mini Analyzer, LI-COR Odyssey XF Imager, and a gentleMACS™ Dissociator. The equipment enabled my team to confirm that repeated HBO2 accelerated fatigue in mice during running and it is not due to a bioenergetic limitation but to calcium trafficking (Figure 1). Additionally, it has improved efficiency and reproducibility in organelle isolation and immunoblotting.
“High-resolution imaging is a pivotal tool in unraveling the intricate mechanisms that govern cellular behavior. By delving into the microscopic realm, we can discern the nuances of how cells and their organelles react and adapt in the face of various stimuli including stress, disease and therapeutic interventions.”
- Heath Gasier, PhD
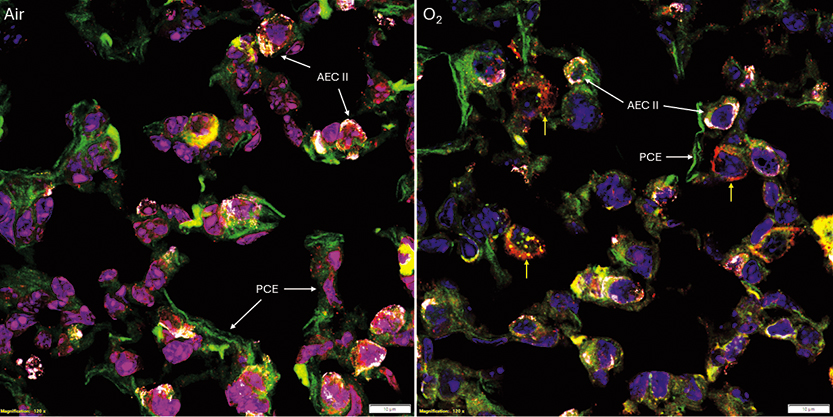
Our group has found that antiepileptic drugs are efficacious in delaying oxygen-induced seizures. Specifically, tiagabine prevents tachycardia, a secondary rise in mean arterial blood pressure, and preserves cerebral blood flow in HBO2. The responses are accompanied by reduced oxidative injury and mitophagy signaling. These findings led to an ONR renewal ($845K) focused on understanding whether tiagabine is efficacious in repeated HBO2 and lowers oxidative brain and lung injury and is related to mitophagy activation. The ability to visualize the location of multiple proteins with high resolution was necessary for aims testing, attainable with a confocal microscope. In 2023, I was awarded a DURIP* award ($242K) for the purchase of a FV3000 Confocal Laser Scanning Microscope with hybrid galvanometer/resonant scanners equipped with four spectral detectors and seven lasers, allowing for multiplexing. The system has made an immediate impact on the labs’ current research and for generating preliminary data in other areas where oxidant production is amplified and impacts mitochondrial turnover, e.g., aging and critical illness. One example is that we have measured mitophagy within alveolar type II cells in a murine model of experimental lung injury to determine its role in recovery (Figure 2).
Joseph Priestly said, “the air which nature has provided for us is as good as we deserve.” While he was correct, breathing a high PO2 is often required in treatment and survival. I am optimistic that my work will not only advance our knowledge but also lead to targeted treatment options that will reduce oxidant stress when exposed to an increased PO2. BP
*DURIP Repair Mechanisms of Oxidative Tissue Damage
Breathing oxygen at increased atmospheric pressure is toxic to the lungs and central nervous system. Breathing oxygen at greater than one atmosphere absolute, HBO2, affects skeletal muscle function that is accompanied by decreased post-dive work performance. Understanding pathological mechanisms of HBO2 is critical for achieving a goal of the United States Navy to safely extend diving operations at greater depths.
Gasier’s latest research aims to determine how HBO2 causes oxidant damage and activates repair mechanisms in tissues and cells using a laser scanning confocal microscope by defining abnormalities in tissue/cellular structure and function initiated by HBO2.
