Lab Description
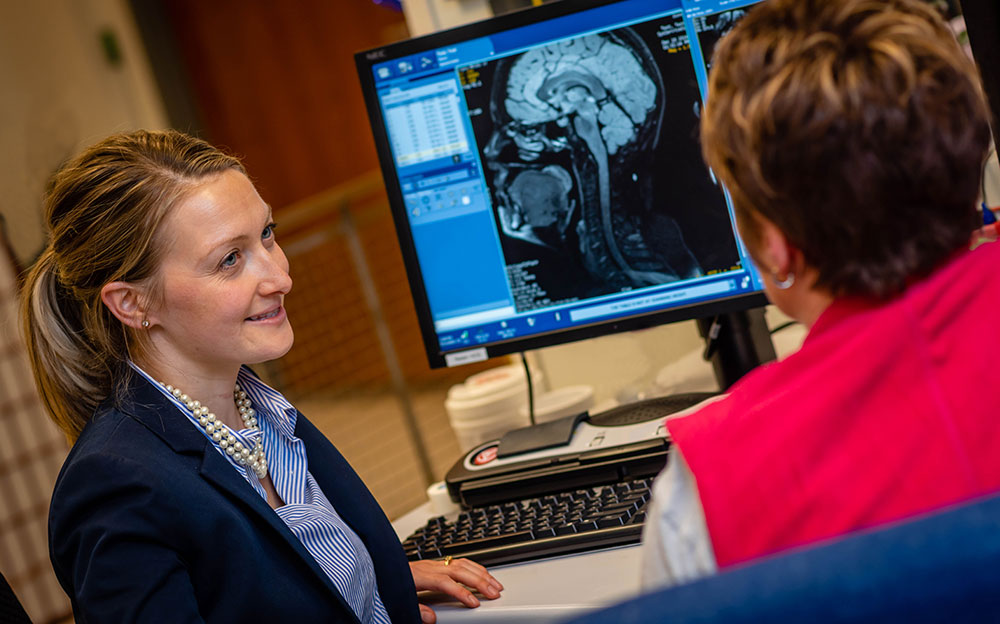
The Human Affect and Pain Neuroscience Laboratory is interested in central nervous system (brain and spinal cord) processes and alterations in individuals with chronic pain. The brain and spinal cord are known to be altered in states of chronic pain, both in structure (or anatomy) and function (or activity). We use a combination of methods using neuroimaging, quantitative sensory testing, and clinical/behavioral testing via questionnaires in our research projects. Our research focuses on questions pertaining to how central nervous system changes are related to chronic pain and associated comorbid symptoms, behavioral changes in chronic pain and opioid use.
Our goal is to contribute impactful data for increased understanding of the mechanisms underlying and contributing to chronic pain by studying the central nervous system in humans (with and without chronic pain) and its relationship to other body systems. Through our research, we hope to provide novel and fundamental knowledge for improved and targeted treatments for individuals suffering with chronic pain.
Currently, we have active clinical research projects studying:
- Alterations in brain and cervical spinal cord structure and function in chronic pain
- The relationship between brain reward systems and behavior in chronic pain
- Neurophysiological effects of opioid medications in chronic pain
Major Research Interests
Altered Brain and Spinal Cord Activity and Structure in Chronic Pain
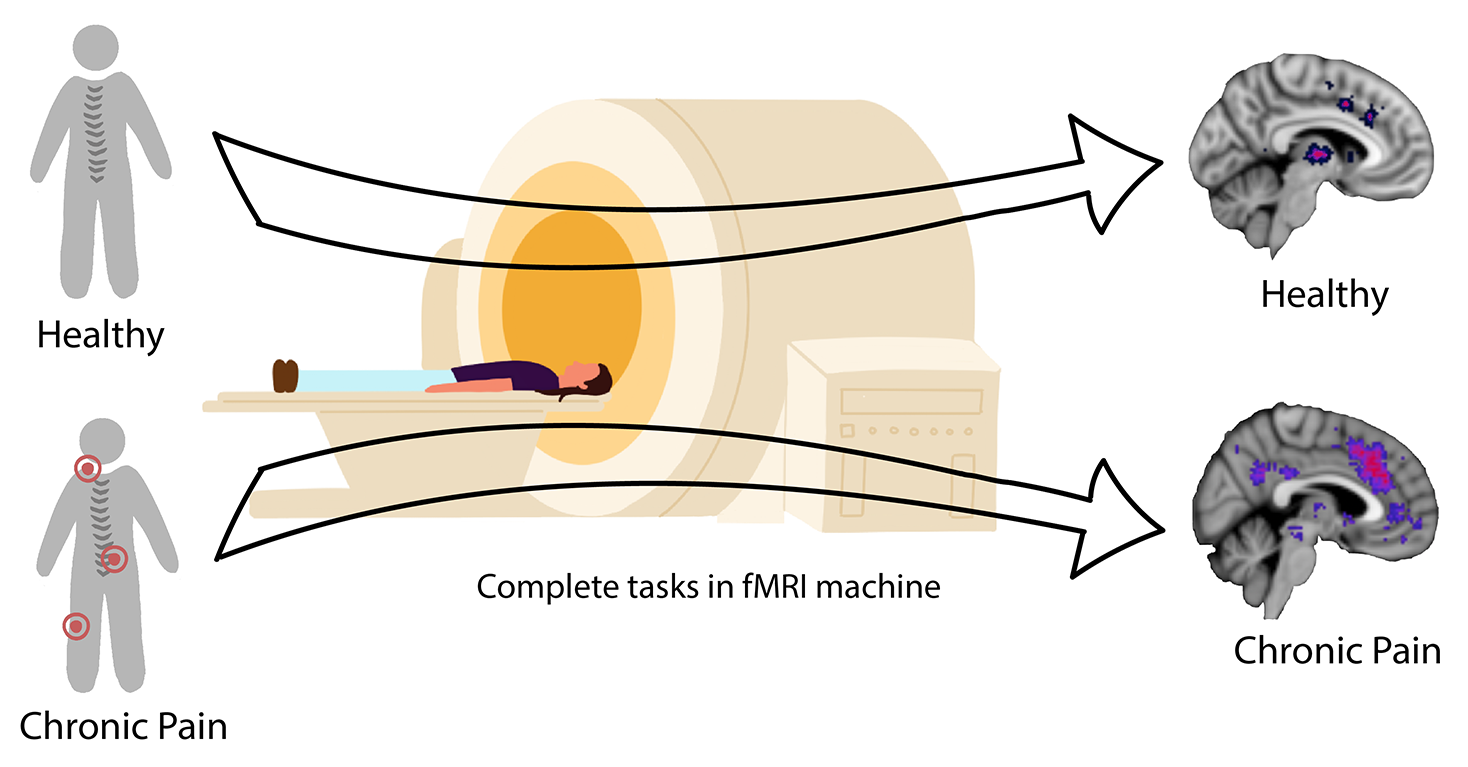
Neuroimaging research has shown over the last few decades that individuals with chronic pain show differences in brain structure and activity. It is thought that these findings represent neurophysiological alterations in the central nervous system that may underlie or contribute to the experience of chronic pain. Our lab uses a technology called magnetic resonance imaging (MRI) to study the brain and spinal cord in individuals with chronic pain. MRI uses a strong magnetic field to create images of the brain and spinal cord that can be used to study both the structure (or anatomy) and function (or activity based on changes in blood flow) without the use of radiation or injections. During these research studies, participants lie down in the MRI scanner and are asked to rest or participate in various tasks (like computer games) while brain and spinal cord images are collected. The images are then analyzed in the laboratory and used to help us understand how changes in the brain and spinal cord may contribute to chronic pain symptoms, so that in turn, we can identify new ways to reverse these changes and new treatments for chronic pain.
Relationship Between Chronic Pain and Reward/Value Systems
Several lines of neuroimaging research indicate that brain reward and value systems are altered in chronic pain. Brain reward and values systems are involved when individuals weigh options and determine courses of action to take. These are highly important processes and are used frequently in daily life. In some individuals with chronic pain, reward and value systems appear to be dysregulated – this could be due either to preexisting abnormalities prior to development of chronic pain or caused by the experience of a constant pain experience. Either way, intact reward and value processes are thought to be critical for proper functioning and daily life, and the disruption of these important processes could, in part, contribute to chronic pain itself through the interaction between psychology and physiology. Because of this, we are studying reward and value systems in individuals with chronic pain compared to healthy pain-free individuals. We hope to better understand how these systems are altered in chronic pain and to find how changes in reward/value systems may be influencing the chronic pain experience. By increasing knowledge in this area, we hope these findings will contribute to the identification of more effective psychological and pharmacological therapies to treat chronic pain.

Effects of Opioid Use on Neurophysiology in Chronic Pain
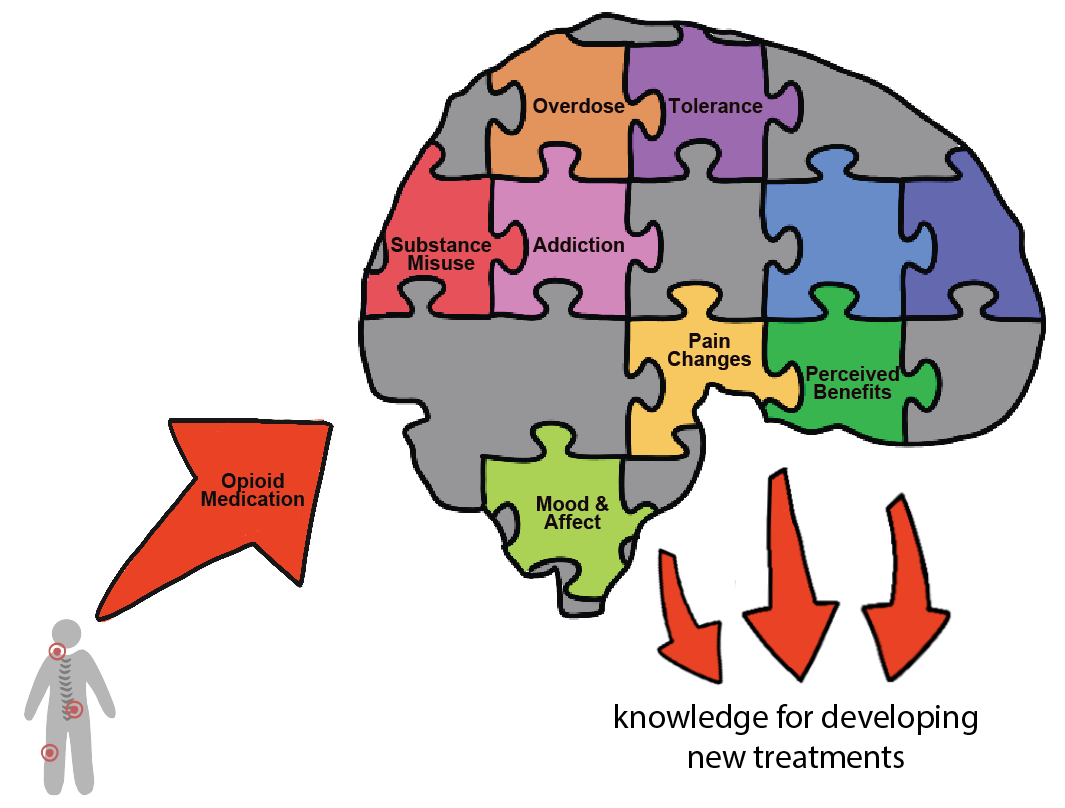
There is currently great controversy over the use of prescription opioids for chronic non-cancer pain. The long-term use of opioids for the treatment of chronic pain has not been shown to benefit or improve chronic pain over time. However, many patients are taking opioid medications and some believe that opioid medications are the only type of medication that effectively reduces their pain (as compared with other types of pain medications). Additionally, opioid medications are associated with many side effects and the potential for serious addiction and overdose. There is very limited evidence of how opioids affect central nervous system neurophysiology (brain structure and activity). We are studying the effects of opioids on central nervous system neurophysiology to determine the positive and/or negative effects of opioids on brain structure and activity. We hope that these findings will, in turn, help inform medical practice regarding the use and prescription of opioids for chronic pain. These research projects are expected to also provide new scientific knowledge that can be used to help develop new pain medications with reduced consequences of addiction and overdose.
Interactions Between Central Nervous System and Other Body Systems in Chronic Pain (Research Collaborations)
The Human Affect and Pain Neuroscience Laboratory primarily studies the central nervous system as a key system involved in and influenced by chronic pain conditions. However, several other major body systems are also involved in and influenced by chronic pain – and these other body systems interact with the central nervous system changes as well. Through collaborations with other researchers and research laboratories, we extend our research on the central nervous system (primarily brain and spinal cord neuroimaging) in order to study how the central nervous system changes interact with other body systems in chronic pain. Current ongoing collaborations include the study of how central nervous system changes are related to altered immune system function in chronic pain.

Lab Members

Lab Alumni















Awards and Recognitions
2025
Congratulations to Drs. Katherine Martucci & Maggie Sweitzer (Duke Psychiatry) on being awarded $3,876,189 for their multi-PI NIH R01 entitled, “Neural correlates and behavioral impact of withdrawal-induced hyperalgesia among people who smoke with and without chronic pain.”
2024
Dr. Katherine Martucci was featured in the September 24, 2024 edition of Duke Medical School’s Magnify magazine in an article titled, "Pain Paradox: How Substance Use May Worsen Chronic Pain."
2023
Brain Implants Have Revealed a Signature for Chronic Pain
By measuring pain signals over months, a new study offers an expansive view of the debilitating condition.
Science News, Katherine Martucci, PhD
Dr. Katherine Martucci was elected to the Board of Directors for the United States Association for the Study of Pain (USASP). This appointment is a 3-year term, during which Dr. Martucci will help to shape and direct the future of the USASP and its mission to promote scientific advances that reduce the burden of pain.
2022

NIH NIDA R01 Awarded to Dr. Katherine Martucci, 2022-2027
In September 2022 the Human Affect and Pain Neuroscience Lab launched its first major R01-funded project! The project entitled “Neurobiological Consequences of Long-Term Opioid Therapy in the Brain and Spinal Cord” will evaluate for the first time how opioids acutely impact the brain and spinal cord activity in individuals with widespread chronic pain conditions.
Varela Research Grant Awarded to HAPN Lab Postdoctoral Research Associate: Congratulations to Dr. Anne Baker, Postdoctoral Research Associate, for her awarded $20,000 “Varela” grant from the Mind & Life Institute. Her project entitled “The Impact of a Mindfulness-Based Intervention on Spinal Cord Activity in Chronic Pain: A Pilot Crossover Study” will study the mechanisms of spinal cord activity under different mindfulness states in individuals with chronic pain.

In the News: As featured by the United States Association for the Study of Pain (USASP)’s “USASP Today” series, Dr. Martucci discusses her journey toward becoming a leader in the field of pain research.

Duke Institute for Brain Sciences (DIBS) Research Incubator Award, Duke University 2022: Congratulations to Dr. Katherine Martucci for being awarded a Duke Institute for Brain Sciences (DIBS) Research Incubator Award. This award provides her and her team with seed funding for a pilot research study on brain changes within the midbrain ventral tegmental area in chronic pain patients who take opioids to develop novel neuro-adaptive treatments for chronic pain.
2021
In the news: Dr. Katherine Martucci’s research on cutting-edge human spinal cord imaging was featured in a special Innovative Research section of the 2021 BluePrint, Duke Anesthesiology’s premier annual publication.

Award to HAPN Lab Postdoctoral Research Associate at Duke Academic Evening, 2021.Congratulations to Dr. Su Hyoun Park, Postdoctoral Research Associate, for her award-recognized presentation at Duke Anesthesiology’s Academic Evening 2021. Her presentation “Altered resting state activity of the brain reward circuit in fibromyalgia” was selected as a finalist for live presentation and awarded 2nd place by guest judge, Dr. Deborah Culley, in the basic science category among several other highly impressive presentations.

Duke Institute for Brain Sciences (DIBS) Research Incubator Award, Duke University 2021: Congratulations to Dr. Katherine Martucci and the faculty of Duke Psychiatry and Behavioral Sciences on their Duke Institute for Brain Sciences (DIBS) Research Incubator Award. The award provides the team with pilot funding to conduct a clinical research study examine the brain’s response to heat pain stimuli among smokers in early withdrawal, to better understand the reasons for increased pain sensitivity.
NIH NIDA Supplement (R00 DA040154-05S1), 2021: Congratulations to Dr. Katherine Martucci on obtaining an administrative supplement from the NIH to promote continuity of biomedical and behavioral research.

DREAM Innovation Grant, Department of Anesthesiology, Duke University School of Medicine, 2020: Congratulations to Dr. Katherine Martucci upon being awarded a DREAM Innovation Grant. Through this award, Dr. Martucci will conduct highly innovative human spinal cord neuroimaging research which will lead to new discoveries and translational research related to spinal cord mechanisms of chronic pain.
International Narcotics Research Conference (INRC) Travel Award, 2019: Congratulations to Dr. Katherine Martucci on winning a travel award to attend and present her work on neurophysiological changes due to opioid use in patients with chronic pain at the 2019 annual International Narcotics Research Conference.
NIH NIDA Pathway to Independence Award (R00), 2018-2021
2018

Dr. Martucci Awarded NIH Grant for Chronic Pain Research
Congratulations to Katherine Martucci, PhD, on receiving a $746,654 R00 grant for her project aimed at characterizing the effects of long-term opioid use on the central nervous system and clinical outcomes in fibromyalgia.
Selected Publications
- Lei C, Park SH, Evington EJ, Rosser MA, Martucci KT. Reduced trapezius pressure pain threshold in fibromyalgia and opioid use. J Pain. 2025 Jun 3;33:105455. doi: 10.1016/j.jpain.2025.105455. Epub ahead of print. PMID: 40472985.
- Baker AK, Nanda M, Park SH, Martucci KT. Attempt to replicate voxel-based morphometry analysis in fibromyalgia: Detection of below threshold differences framed by contributions of variable clinical presentation to low reproducibility. medRxiv, version posted March 8, 2022. doi: 10.1101/2022.03.04.22271900.
- Park SH, Deng EZ, Baker AK, MacNiven KH, Knutson BK, Martucci KT. Altered brain reward response to monetary incentives in fibromyalgia: A replication study. medRxiv, version posted March 3, 2022. doi: 10.1101/2022.03.02.22271367.
- Park SH, Baker AK, Krishna V, Martucci KT. Intact corticostriatal and altered subcortical circuits in chronic pain. medRxiv, version posted September 13, 2021. doi: 10.1101/2021.09.08.21263285.
- Martucci KT, Weber KA 2nd, Mackey SC. Spinal Cord Resting State Activity in Individuals With Fibromyalgia Who Take Opioids. Front Neurol. 2021;12:694271. doi: 10.3389/fneur.2021.694271. eCollection 2021. PubMed PMID: 34421798; PubMed Central PMCID: PMC8371264.
- Karshikoff B, Martucci KT, Mackey S. Relationship Between Blood Cytokine Levels, Psychological Comorbidity, and Widespreadness of Pain in Chronic Pelvic Pain. Front Psychiatry. 2021;12:651083. doi: 10.3389/fpsyt.2021.651083. eCollection 2021. PubMed PMID: 34248700; PubMed Central PMCID: PMC8267576.
- Martucci KT, MacNiven KH, Borg N, Knutson B, Mackey SC. Apparent Effects of Opioid Use on Neural Responses to Reward in Chronic Pain. Sci Rep. 2019 Jul 3;9(1):9633. doi: 10.1038/s41598-019-45961-y. PubMed PMID: 31270360; PubMed Central PMCID: PMC6610070.
- Martucci KT, Weber KA 2nd, Mackey SC. Altered Cervical Spinal Cord Resting-State Activity in Fibromyalgia. Arthritis Rheumatol. 2019 Mar;71(3):441-450. doi: 10.1002/art.40746. Epub 2019 Feb 11. PubMed PMID: 30281205; PubMed Central PMCID: PMC6393192.
- Martucci KT, Borg N, MacNiven KH, Knutson B, Mackey SC. Altered prefrontal correlates of monetary anticipation and outcome in chronic pain. Pain. 2018 Aug;159(8):1494-1507. doi: 10.1097/j.pain.0000000000001232. PubMed PMID: 29790868; PubMed Central PMCID: PMC6053327.
- Jarrahi B, Martucci KT, Nilakantan AS, Mackey S. Cold Water Pressor Test Differentially Modulates Functional Network Connectivity in Fibromyalgia Patients Compared with Healthy Controls. Annu Int Conf IEEE Eng Med Biol Soc. 2018 Jul;2018:578-582. doi: 10.1109/EMBC.2018.8512350. PubMed PMID: 30440463; PubMed Central PMCID: PMC6267987.
- Martucci KT, Mackey SC. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology. 2018 Jun;128(6):1241-1254. doi: 10.1097/ALN.0000000000002137. Review. PubMed PMID: 29494401; PubMed Central PMCID: PMC5953782.
- Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017 Jun;158(6):1069-1082. doi: 10.1097/j.pain.0000000000000886. PubMed PMID: 28328579; PubMed Central PMCID: PMC5435510.
- Huang L, Kutch JJ, Ellingson BM, Martucci KT, Harris RE, Clauw DJ, Mackey S, Mayer EA, Schaeffer AJ, Apkarian AV, Farmer MA. Brain white matter changes associated with urological chronic pelvic pain syndrome: multisite neuroimaging from a MAPP case-control study. Pain. 2016 Dec;157(12):2782-2791. doi: 10.1097/j.pain.0000000000000703. PubMed PMID: 27842046; PubMed Central PMCID: PMC5117992.
- Martucci KT, Shirer WR, Bagarinao E, Johnson KA, Farmer MA, Labus JS, Apkarian AV, Deutsch G, Harris RE, Mayer EA, Clauw DJ, Greicius MD, Mackey SC. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP Research Network. Pain. 2015 Sep;156(9):1755-1764. doi: 10.1097/j.pain.0000000000000238. PubMed PMID: 26010458; PubMed Central PMCID: PMC4545714.
- Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Apkarian VA, Mayer EA, Clauw DJ, Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain. 2014 Dec;155(12):2502-2509. doi: 10.1016/j.pain.2014.09.002. Epub 2014 Sep 19. PubMed PMID: 25242566; PubMed Central PMCID: PMC4504202.
- Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain. 2014 Dec;155(12):2491-2501. doi: 10.1016/j.pain.2014.07.008. Epub 2014 Jul 15. PubMed PMID: 25047783; PubMed Central PMCID: PMC4250429.
- Zeidan F, Martucci KT, Kraft RA, McHaffie JG, Coghill RC. Neural correlates of mindfulness meditation-related anxiety relief. Soc Cogn Affect Neurosci. 2014 Jun;9(6):751-9. doi: 10.1093/scan/nst041. Epub 2013 Apr 24. PubMed PMID: 23615765; PubMed Central PMCID: PMC4040088.
- Martucci KT, Eisenach JC, Tong C, Coghill RC. Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. Pain. 2012 Jun;153(6):1232-1243. doi: 10.1016/j.pain.2012.02.035. Epub 2012 Apr 13. PubMed PMID: 22503222; PubMed Central PMCID: PMC3358522.
- Martucci KT, Yelle MD, Coghill RC. Differential effects of experimental central sensitization on the time-course and magnitude of offset analgesia. Pain. 2012 Feb;153(2):463-472. doi: 10.1016/j.pain.2011.11.010. Epub 2011 Dec 6. PubMed PMID: 22154333; PubMed Central PMCID: PMC3264887.
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011 Apr 6;31(14):5540-8. doi: 10.1523/JNEUROSCI.5791-10.2011. PubMed PMID: 21471390; PubMed Central PMCID: PMC3090218.
- Martucci KT, Coghill RC. Regional anesthesia: functional implications beyond the anesthetized nerve. Anesthesiology. 2011 Jan;114(1):21-3. doi: 10.1097/ALN.0b013e3182016521. PubMed PMID: 21150575.
Photos

Left to Right: Amy Westerhoff, Dr. Sadé Orejobi, Dr. Su Hyoun Park, Dr. Katherine Martucci, Emily Bell, Justine Prophete





Left to Right: Eden Deng, Dr. Su Hyoun Park, Dr. Anne Baker, Dr. Katherine Martucci













