
2012 Final DIG Report | “Effect of an Mn-porphyrin in Neuropathic Pain”
Clinical investigations indicate that 60% to 80% of spinal cord injury (SCI) patients experience pain, which is severe in nature in at least one-third of them. My DIG research was to examine the efficacy of Mn-porphyrin, a catalytic oxidoreductant, in treating SCI-induced neuropathic pain in mice, and explore its potential for therapeutic development.
Our laboratory has a long history of studying central nervous system (CNS) injury, but neuropathic pain was new to us. With the support of the DIG, we were able to establish the animal models, purchase the equipment for behavioral tests, and do preliminary experiments; allowing expansion of our research to a new domain.
Findings
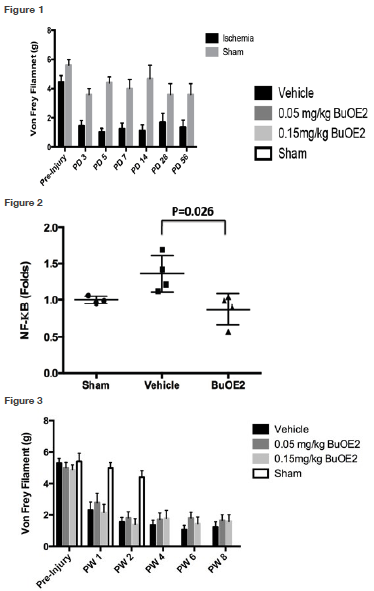
The first experiment explored spinal cord ischemia, a common complication from surgical repair of thoracoabdominal aortic aneurysms. Mice were subjected to either 10 minutes of thoracic aorta clamping or sham surgery, and then observed for pain-like behavioral responses. The injured mice that were subject to clamping became less tolerant to fine von Frey filament stimulus, compared to sham surgery mice (p=0.02, fig. 1), which was consistent with the development of neuropathic pain. This response persisted for eight weeks and beyond, and represented a new model for studying this disorder.
Next, we examined whether the Mn-porphyrin, BuOE2, decreased the activity of NF-kB, a key transcription factor in the inflammation pathway. Mice were subjected to 10 minutes of clamping of the thoracic aorta, and then received 0.25 mg/kg BuOE2 or saline. The NF-kB activity was measured at 24 hours post-injury. We found that the BuOE2 suppressed SCI-induced NF-kB activity (p=0.03, fig. 2).
Our second experiment was related to lumbar spinal cord injury, a condition of acute traumatic injury or a central type of lumbar disc herniation. Mice were subjected to left lumbar spinal cord hemisection and then received saline, 0.05 and 0.15 mg/kg BuOE2, one hour after injury, twice per day, for two weeks. As a normal reference, 10 mice then received sham surgery. Pain-like responses were observed weekly for eight weeks. After the eight-week period, we observed that injury-induced neuropathic pain was slightly reduced by BuOE2 (fig. 3), which was not statistically significant due to low drug dose or severe hemisection.
Next Steps
To further explore BuOE2 efficacy, we plan to try a higher dose of the compound and investigate a spinal cord contusion injury model. We are hopeful that this will allow us to collect sufficient data for a translational NIH R21 proposal.
