Lab Description

Chronic pain is a major public health issue in the United States, affecting approximately 100 million Americans. Despite its widespread impact, current treatment options remain largely inadequate. The ongoing opioid epidemic underscores the urgent need for more effective and safer pain therapies.
The primary goal of our laboratory is to uncover novel molecular and cellular mechanisms that drive the transition from acute to chronic pain. We employ a multidisciplinary approach that integrates in vitro, ex vivo, and in vivo methods, including electrophysiology and behavioral assessments in animal models of neuropathic, postoperative, inflammatory, and cancer-related pain, as well as acute and chronic itch. We also utilize human cellular and tissue models to investigate pain mechanisms and evaluate potential therapeutics. Our research has revealed critical neuro-immune interactions that contribute to pain pathogenesis and resolution.
We are dedicated to advancing the understanding of pain mechanisms with the ultimate goal of developing innovative, non-opioid therapies to prevent and treat acute and chronic pain.
Lab Opportunities
The Ji lab is always recruiting talented postdoctoral fellows, visiting scholars, graduate students, and undergraduate students. Interested candidates should email their CV, a brief statement of interests, and a list of three references.
Major Research Interests
Investigate Protective and Detrimental Roles of Glial Cells in the Onset and Resolution of Pain
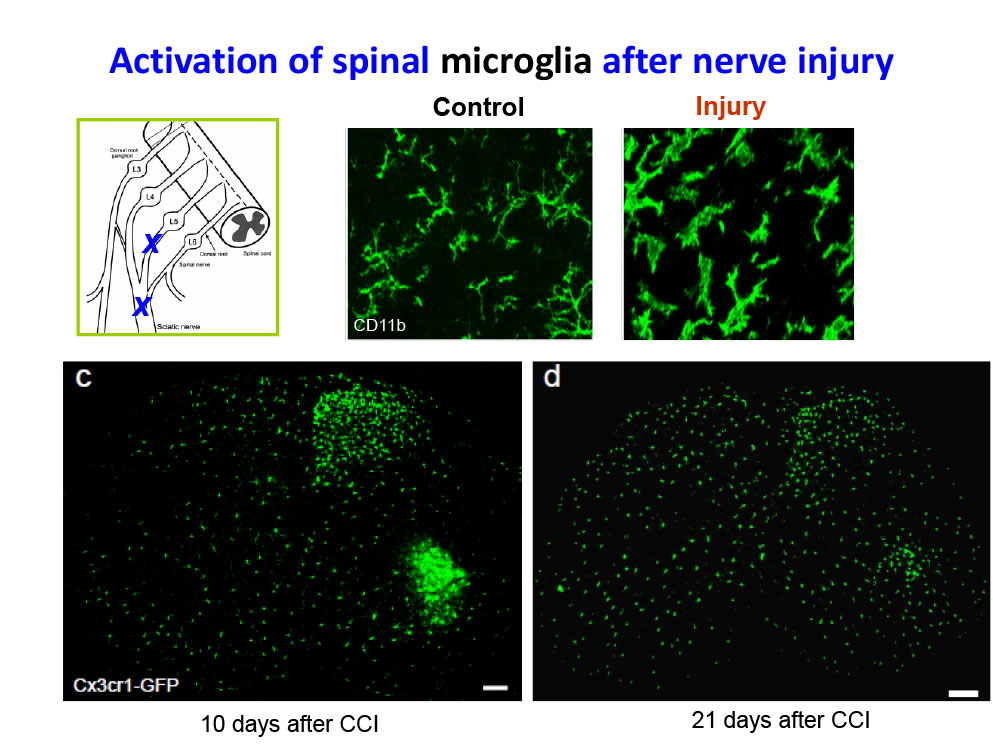
Specifically, we study how astrocytes and microglia in the spinal cord, as well as satellite glial cells in the dorsal root ganglia, regulate pain through neuro-glial interactions. In addition, we examine the roles of macrophages, cancer cells, and stem cells in modulating pain responses.
Study of Human Sensory Neurons and Glial Cells
We utilize human dorsal root ganglion (DRG) sensory neurons from both diseased and non-diseased donors to investigate pain mechanisms and evaluate potential treatments in a clinically relevant in vitro model (PMID: 28424991, PMID:27916453, PMID:26479925, PMID: 38530364).
Development of Non-Opioid Pain Therapeutics for the Treatment of Acute and Chronic Pain
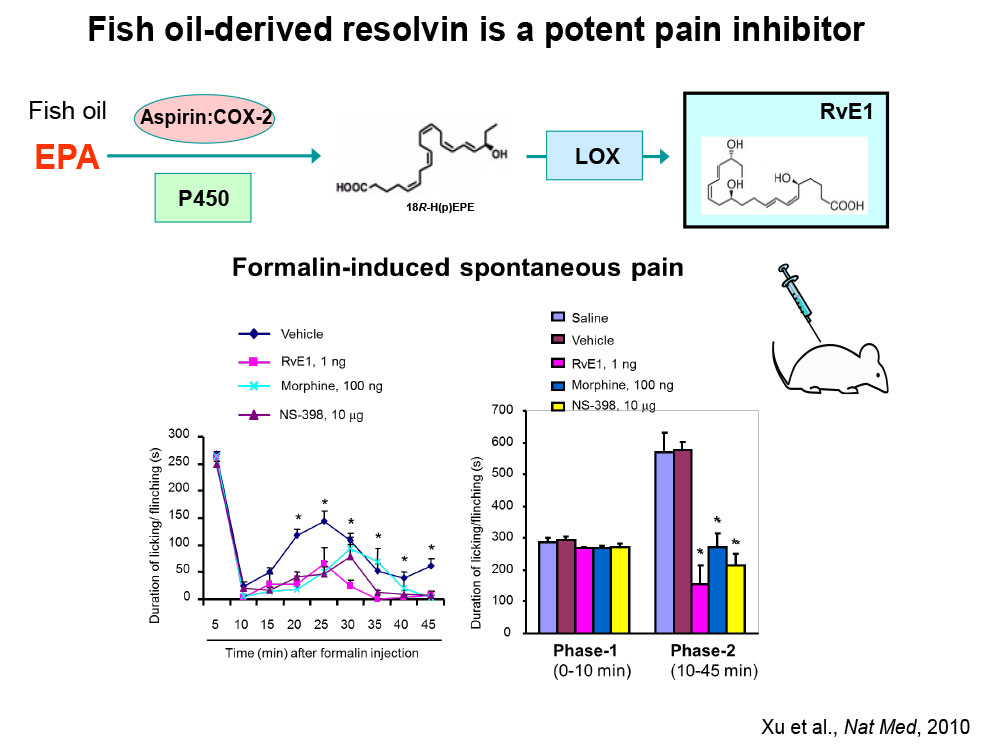
Specialized Proresolving Mediators (SPMs) and their analogs: We investigate how SPMs —such as resolvins, protectins, and maresins—and their synthetic analogs alleviate pain and itch (PMID: 20383154; PMID: 36100219).
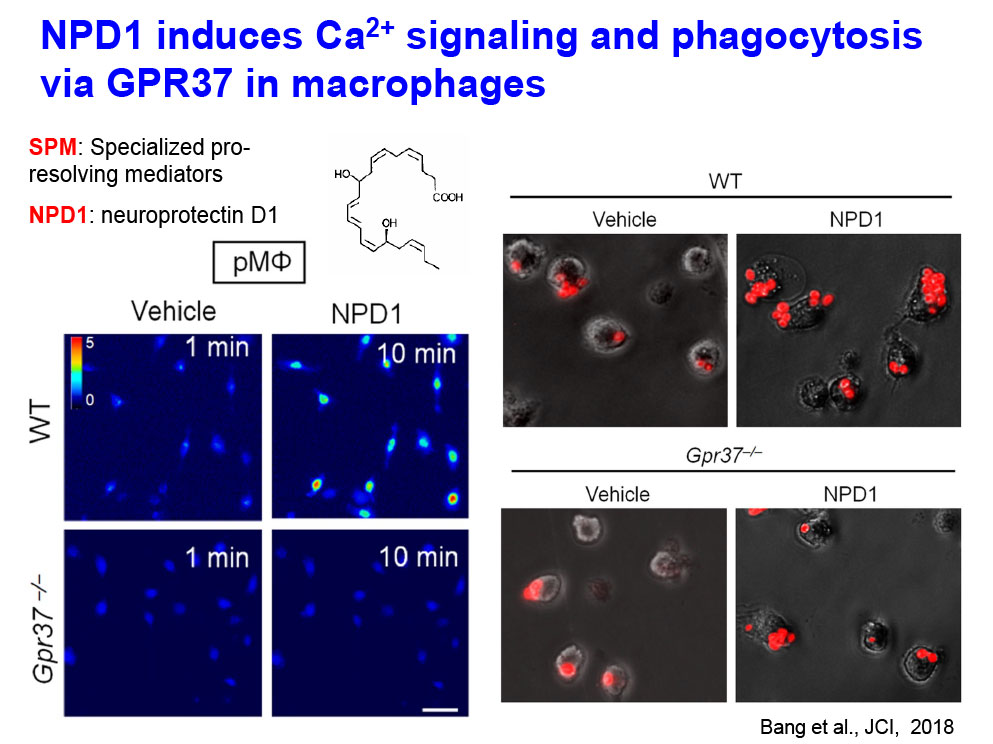
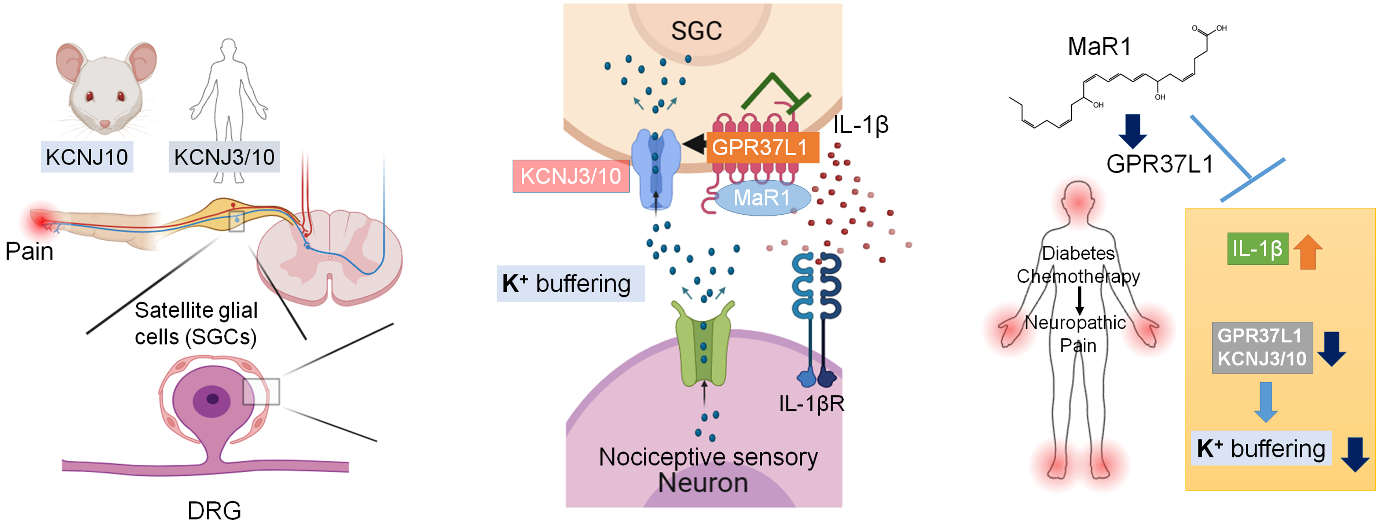
SPM receptors (GPR37 and GPR37L1): We investigate how GPR37 and GPR37L1 can protect against neuropathic pain after diabetes and chemotherapy (PMID: 30010619; PMID: 38530364; PMID: 39952243).
Arrestin-biased allosteric modulator of neurotensin receptor 1 (PMID: 40393456). SBI-810 alleviates acute and chronic pain (Guo et al., Cell, 2025)
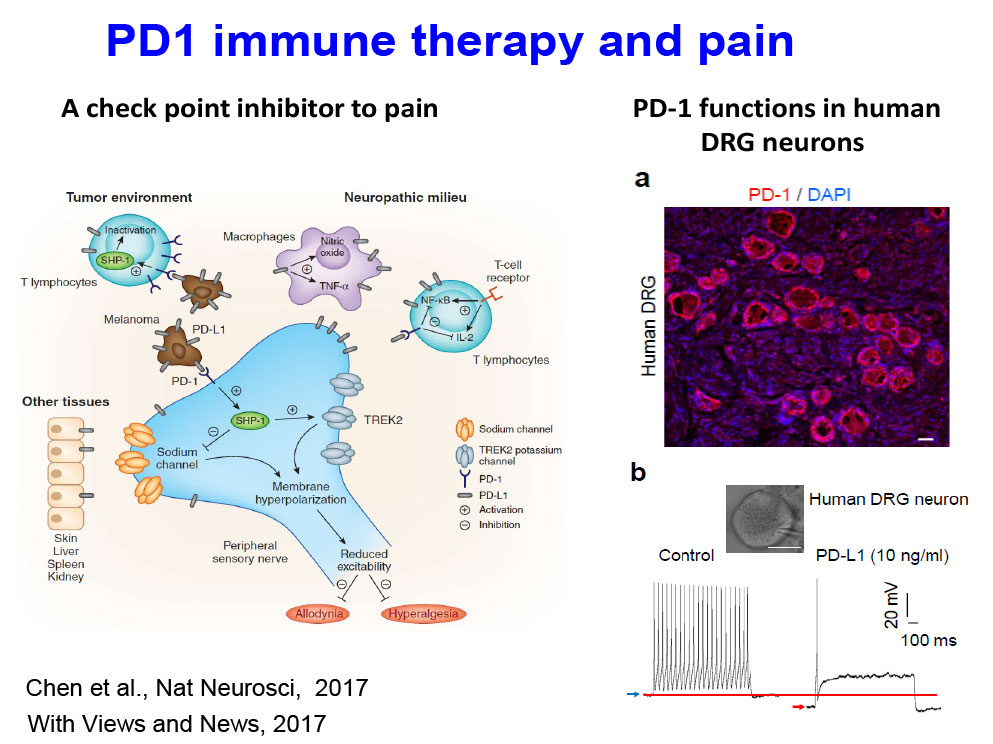
Immunotherapy for chronic pain: We investigate PD-1– and STING-based immunotherapies for the treatment of cancer pain and chronic pain (PMID: 32484460; PMID: 34315904).
Regenerative medicine. We investigate long-term pain relief using mesenchymal stromal cells (PMID: 26168219) and autologous conditioned serum (ACS, PMID: 37150265), focusing on their mechanisms of action.
Neuromodulation: We study how neuromodulation techniques, such as spinal cord stimulation (SCS) and auricular stimulation, promote long-term pain relief through neuro-immune modulation.
Chronic Itch in Cutaneous T-cell Lymphoma (CTCL): We have developed a mouse model of CTCL characterized by severe chronic itch and are using this model to evaluate novel therapeutic strategies.

Springer Book
Editors: Ru-Rong Ji, Jianguo Cheng, and Jasmine Ji
Inflammation, Pain, and Neuroimmune Interactions.
Springer Nature, Published on May 7, 2023 (16 chapters, 364 pages)
Development of Novel Pain Therapeutics and Diagnosis for the Translation from Bench to Bed
- Specialized Proresolving Mediators (SPMs) [PMID: 30010619]: We investigate how SPMs, such as neuroprotectin D1 (NPD1) alleviate pain via specific receptors (e.g., GPR37) and distinct mechanisms (phagocytosis).
- Stem cells [PMID: 26168219]: We are testing new methods that can enhance the homing and analgesic efficacy of bone marrow stem cells.
- Immune therapy and cancer pain [PMID 28530662]: We examine how PD-L1 and PD-1 regulate neuronal activity and cancer pain.
- Neuromodulation: We investigate how neuromodulation such as electroacupuncture and auricular stimulation can produce long-term pain relief via regulation of neuro- inflammation.
- Human sensory neurons [PMID: 28424991, 27916453, 26479925]: We use human DRG neurons from donors to test mechanisms and treatments of clinical pain in “a dish”.
Immune Checkpoint Inhibitors in the Nervous System
[PMID 28530662]
We examine how PD-L1 and PD-1 regulate neuronal activity in the PNS and CNS.
Resolution Mechanisms and Mediators of Pain
[PMID: 20383154, PMID: 21963090]
One of the key mechanisms for the transition from acute pain to chronic pain is a failure in the resolution of acute pain and acute inflammation.
- We investigate how specialized pro-resolution mediators (SPMs), such as resolvins, neuroprotectins, and marresins, derived from omega-3 unsaturated fatty acids DHA and EPA, control pain by regulating inflammation, glial activation, TRP channels, and synaptic plasticity [PMID: 22171045].
Lab Members
In the News
May 30, 2025
The Indian Practitioner (IN)
The Future of Painkillers: Powerful, Safe, and Non-Addictive
May 19, 2025
Duke University School of Medicine
Experimental Painkiller Could Outsmart Opioids – Without the High
May 20, 2025
The Duke Daily
Painkiller could offer relief without dangerous side effects of opioids
ScienceBlog
New Pain Drug Targets Nerve Receptors, Bypassing Addiction Pathway
Daily Express
Scientists Invent Powerful New Painkiller with Fewer Side Effects
Science Daily
Experimental painkiller could outsmart opioids -- without the high
Medical Xpress on MSN.com
Experimental painkiller could outsmart opioids—without the high
The University Network
Revolutionary Non-Opioid Painkiller: A Game Changer in Pain Management
Apr 29, 2025
United States Association for the Study of Pain
Dr. Ru-Rong Ji elected as 2025 Fellows | USASP
Apr 16, 2025
Neuron Editor's Pick
Astrocytic GPR37L1: A New Guardian Against the Onset and Chronicity of Neuropathic Pain
Mar 24, 2025
Cell Res Editor's Pick
Nociceptor Neurons Promote PDAC Progression and Cancer Pain by Interaction with Cancer-Associated Fibroblasts and Suppression of Natural Killer Cells
Feb 11, 2025
Neuron Editor's Pick
GPR37L1 Identifies Spinal Cord Astrocytes and Protects Neuropathic Pain After Nerve Injury
December 3, 2024
Duke Science & Technology LAUNCH Fund
May 29, 2024
Shirley Morton
Dean’s Staff Award Winners Named | Duke University School of Medicine
March 2024
March SCI IMMUNOL Editor's Pick
New Publication highlighted by IASP Pain Research Forum
Jan 6, 2024
BRAIN BEHAV IMMUN Editor's Pick
New Publication highlighted by IASP Pain Research Forum
Dec 4, 2023
Nov 15, 2023
Most Highly Cited: 30 for '23 - Research Blog (duke.edu)
Nov 11, 2023
Investigators to Share New Understanding of Chronic Pain in Rheumatic Disease
Oct 5, 2023
Dr. Ji Awarded NIH Grant to Study Checkpoint Inhibitors for Pain Control
May 9, 2023
ACS Paper Published in Brain, Behavior, and Immunity and Noticed on Twitter
May 7, 2023
Neuroimmune Interactions in Pain: Mechanisms and Therapeutics | SpringerLink
May 6, 2023
New Publication highlighted by IASP Pain Research Forum
March 13, 2023
Key Role Identified for Nervous System in Severe Allergic Shock | Duke Health
November 2022
March 2022
The Physiology of Pain | American Physiological Society
December 23, 2021
The Most Popular PRF News Story of 2021 Explored the Discovery of a Sex-Specific Pain Mechanism
December 17, 2021
STING Immunotherapy: A Complementary Approach to Treating Bone Cancer Pain
November 23, 2021
Dr. Ji Named a Highly Cited Researcher
November 12, 2021
Sex-Specific Differences in Pain Mechanism
October 27, 2021
June 23, 2021
High-Fat Diet Can Cause Chronic Pain, Bariatric News, Aidan McGinnis, Ru-Rong Ji
February 1, 2021
November 20, 2020
Dr. Ji Named a Highly Cited Researcher
November 19, 2020
Dr. Ji is Among One of 37 Highly-Cited-Duke Investigators
July 21, 2020
Dr. Ji Receives 2020 American Society of Anesthesiologists (ASA) Excellence in Research Award
June 5, 2020
Paper’s of the Week, Editor’s pick, Pain Research Forum
May 11, 2020
January 23, 2020
Dr. Ji Receives Founders Award from the American Academy of Pain Medicine
December 19, 2019
Duke Today: Cryo-Electron Microscope Captures Details of the ‘Wasabi Sensor’
November 19, 2019
Global List of Highly Cited Puts Duke in Top Ten
December 3, 2018
Anesthesiologist Named a Highly Cited Researcher for 2018
August 13, 2018
Tiny Bits of RNA Can Trigger Pain and Itchiness - Science News
August 8, 2018
Small RNAs, but Sizable Itch: TRPA1 Activation by an Extracellular MicroRNA - Science Direct
Inflammatory Pain: Be It Resolved! - Pain Research Forum
June 30, 2018
Ji lab fellow awarded John J. Bonica Fellowship from International Association for the Study of Pain
November 8, 2017
Star-Shaped Brain Cells Orchestrate Neural Connections - Duke Today
June 7, 2017
How Cancers Mask Pain so Tumors Can Grow Unnoticed - STAT
May 23, 2017
Immunotherapy Target Suppresses Pain to Mask Cancer - Duke Today
May 22, 2017
New painkiller discovered in cancer cells - The San Diego Union
February 27, 2017
School of Medicine Recognizes “Noteworthy” Faculty
Endowed Professorships - Duke Anesthesiology
January 4, 2017
Autism Gene Modulates Thermal Pain - Pain Research Forum
December 1, 2016
Autism-Linked Protein Crucial for Feeling Pain - ScienceDaily
Autism-Linked Protein Crucial For Feeling Pain - Duke Today
November 14, 2016
Dr. Ji Published in Special Issue of Science
November 5, 2015
Targeting Toll-like receptors to treat chronic pain - Nature Medicine
December 1, 2015
Pain: TLR5 opens the door to neuropathic-pain treatment. - NCBI
August 11, 2015
Stem Cells Quell Neuropathic Pain in Mice - Pain Research Forum
July 13, 2016
Pain Relief Breakthrough: Experimental Stem Cell Procedure - Medical Daily
Stem Cells Provide Lasting Pain Relief in Mice - NIH
Stem Cells Provide Lasting Pain Relief in Mice - Duke Today
October 28, 2014
Diverse Interests in the Basic Science of Pain: A Conversation With Ru-Rong Ji - Pain Research Forum
July 17, 2013
Uncovering a Healthier Remedy for Chronic Pain - Duke Today
May 7, 2012
Duke Announces 2012 Distinguished Professors - Duke Today
Selected Publications
Selected Recent Publications at Duke (Since 2012)
- Guo R, Chen O, Zhou Y, Li Y, Bang S, Chandra S, Chen G, Xie R, He W, Xu J, Zhou R, Person K, Moore MN, Alwin AR, Spasojevic I, Jackson M, Olson SH, Caron M, Slosky LM, Wetsel WC, Barak L, Ji RR. Arrestin-biased allosteric modulator of neurotensin receptor reduces acute and chronic pain without abusive lability. Cell, 2025 May 15:S0092-8674(25)00508-2.
- Xu J, Yan Z, Gang S, Velmeshev D, Ji RR. GPR37L1 identifies spinal cord astrocytes and protects neuropathic pain after nerve injury. Neuron, 2025, Neuron. Apr 16;113(8):1206-1222.e6.
- Wright NJ, Matsuoka Y, Park H, He W, Webster CG, Furutani K, Fedor JG, McGinnis A, Zhao Y, Chen O, Bang S, Fan P, Spasojevic I, Hong J*, Ji RR*, Lee SY*. Design of an equilibrative nucleoside transporter subtype 1 inhibitor for pain relief. Nat Commun. 2024 Dec 30;15(1):10738.
- Stinson NC, Matsuoka Y, Agarwal A, Dziewior CS, McDonald SM, Li Y, Godwin K, Ji RR, Becker ML. Pre-Clinical Assessment of Bupivacaine-Loaded Poly(ester urea) Thin Films for Controlled Drug Release and Effective Pain Management After Surgery. Adv Healthc Mater. 2024 Dec 12:e2402800. doi: 10.1002/adhm.202402800.
- Hayes BW, Choi HW, Rathore APS, Bao C, Shi J, Huh Y, Kim MW, Mencarelli A, Bist P, Ng LG, Shi C, Nho JH, Kim A, Yoon H, Lim D, Hannan JL, Purves JT, Hughes FM Jr, Ji RR, Abraham SN. Recurrent infections drive persistent bladder dysfunction and pain via sensory nerve sprouting and mast cell activity. Sci Immunol. 2024 Mar;9(93):eadi5578.
- Bang S, Jiang C, Xu J, Chandra S, McGinnis A, Luo X, He Q, Li Y, Wang Z, Ao X, Parisien M, Oliveira Fernandes de Araujo L, Jahangiri Esfahani S, Zhang Q, Tonello R, Berta T, Diatchenko L, Ji RR. Satellite glial GPR37L1 and its ligand maresin 1 regulate potassium channel signaling and pain homeostasis. J Clin Invest. 2024 Mar 26;134(9):e173537.
- Ji RR. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu Rev Pharmacol Toxicol. 2023 Jan 20; 63:273-293. doi: 10.1146/annurev-pharmtox-051921-084047.
- Bao C, Chen O, Sheng H, Zhang J, Luo Y, Hayes BW, Liang H, Liedtke W, Ji RR, Abraham SN. A mast cell-thermoregulatory neuron circuit axis regulates hypothermia in anaphylaxis. Sci Immunol. 2023 Mar 17;8(81): eadc9417. doi: 10.1126/sciimmunol. adc9417.
- Buchheit T, Huh Y, Breglio A, Bang S, Xu J, Matsuoka Y, Guo R, Bortsov A, Reinecke J, Wehling P, Jun Huang T, Ji RR. Intrathecal administration of conditioned serum from different species resolves Chemotherapy-Induced neuropathic pain in mice via secretory exosomes. Brain Behav Immun. 2023 May 5; 111:298-311.
- Zhao J, Huh Y, Bortsov A, Diatchenko L, Ji RR. Immunotherapies in chronic pain through modulation of neuroimmune interactions. Pharmacol Ther. 2023 Aug; 248:108476.
- Zhao J, Bang S, Furutani K, McGinnis A, Jiang C, Roberts A, Donnelly CR, He Q, James ML, Berger M, Ko MC, Wang H, Palmiter RD, Ji RR. PD-L1/PD-1 checkpoint pathway regulates hippocampal neuronal excitability and learning and memory behavior. Neuron. 2023 Sep 6;111(17):2709-2726.e9.
- Chen O, He Q, Han Q, Furutani K, Gu Y, Olexa M, Ji RR. Mechanisms and treatments of neuropathic itch in a mouse model of lymphoma. J Clin Invest. 2023 Feb 15;133(4):e160807. doi: 10.1172/JCI160807.
- Liao Y, Ren Y, Luo X, Mirando AJ, Long JT, Leinroth A, Ji RR, Hilton MJ. Interleukin-6 signaling mediates cartilage degradation and pain in posttraumatic osteoarthritis in a sex-specific manner. Sci Signal. 2022 Jul 26;15(744): eabn7082.
- Chen G, Xu J, Luo H, Luo X, Singh SK, Ramirez JJ, James ML, Mathew JP, Berger M, Eroglu C, Ji RR. Hevin/Sparcl1 drives pathological pain through spinal cord astrocyte and NMDA receptor signaling. JCI Insight. 2022 Dec 8;7(23):e161028.
- Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron. 2021 Sep 1;109(17):2691-2706.e5.
- Baldwin KT, Tan CX, Strader ST, Jiang C, Savage JT, Elorza-Vidal X, Contreras X, Rülicke T, Hippenmeyer S, Estévez R, Ji RR, Eroglu C. HepaCAM controls astrocyte self-organization and coupling. Neuron. 2021 Aug 4;109(15):2427-2442.e10.
- Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, Tao X, Bang S, McGinnis A, Lee M, Hilton MJ, Ji RR. STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun. 2021 Jul 27;12(1):4558.
- Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, Ding H, Zhao J, Luo X, Lee MS, Lei YL, Maixner W, Ko MC, Ji RR. STING controls nociception via type I interferon signalling in sensory neurons. Nature. 2021 Mar;591(7849):275-280.
- Wang Z, Jiang C, Yao H, Chen O, Rahman S, Gu Y, Zhao J, Huh Y, Ji RR. Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain. 2021 Mar 3;144(2):665-681.
- Deerhake ME, Danzaki K, Inoue M, Cardakli ED, Nonaka T, Aggarwal N, Barclay WE, Ji RR, Shinohara ML. Dectin-1 limits autoimmune neuroinflammation and promotes myeloid cell-astrocyte crosstalk via Card9-independent expression of Oncostatin M. Immunity. 2021 Mar 9;54(3):484-498.e8.
- Bang S, Donnelly CR, Luo X, Toro-Moreno M, Tao X, Wang Z, Chandra S, Bortsov AV, Derbyshire ER, Ji RR. Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat Commun. 2021 Mar 17;12(1):1704.
- Brigham NC, Ji RR, Becker ML. Degradable polymeric vehicles for postoperative pain management. Nat Commun. 2021 Mar 1;12(1):1367.
- Wang Z, Jiang C, He Q, Matsuda M, Han Q, Wang K, Bang S, Ding H, Ko MC, Ji RR. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci Transl Med. 2020 Feb 19;12(531).
- Suo Y, Wang Z, Zubcevic L, Hsu AL, He Q, Borgnia MJ, Ji RR, Lee SK. Structural insights into electrophile irritant sensing by the human TRPA1 channel. Neuron, 2019, 2020 Mar 4;105(5):882-894.
- Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 2019 Nov;20(11):667-685.
- Lopez T, Mustafa Z, Chen C, Lee KB, Ramirez A, Benitez C, Luo X, Ji RR, Ge X. Functional selection of protease inhibitory antibodies. Proc Natl Acad Sci U S A. 2019 Aug 13;116(33): 16314-16319.
- Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, Ji RR. Macrophage Toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J Neurosci. 2019 Aug 28;39(35):6848-6864.
- Chamessian A, Matsuda M, Young M, Wang M, Zhang ZJ, Liu D, Tobin B, Xu ZZ, Van de Ven T, Ji RR. Is Optogenetic activation of Vglut1-positive Aβ low-threshold mechanoreceptors sufficient to induce tactile allodynia in mice after nerve injury? J Neurosci. 2019 Jul 31;39(31):6202-6215.
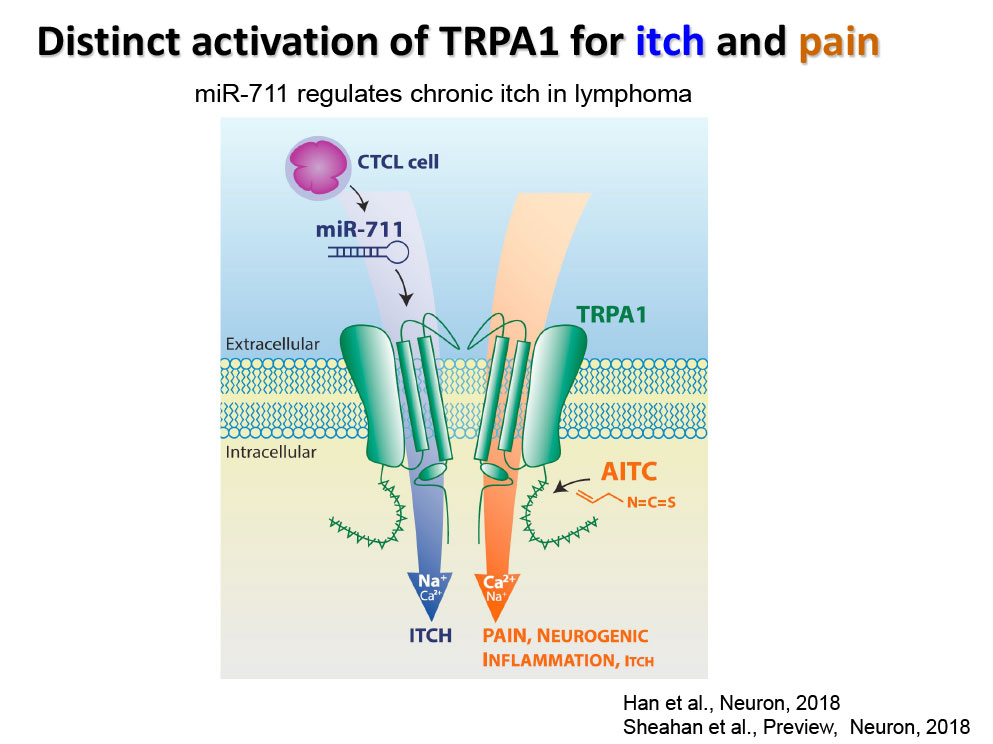
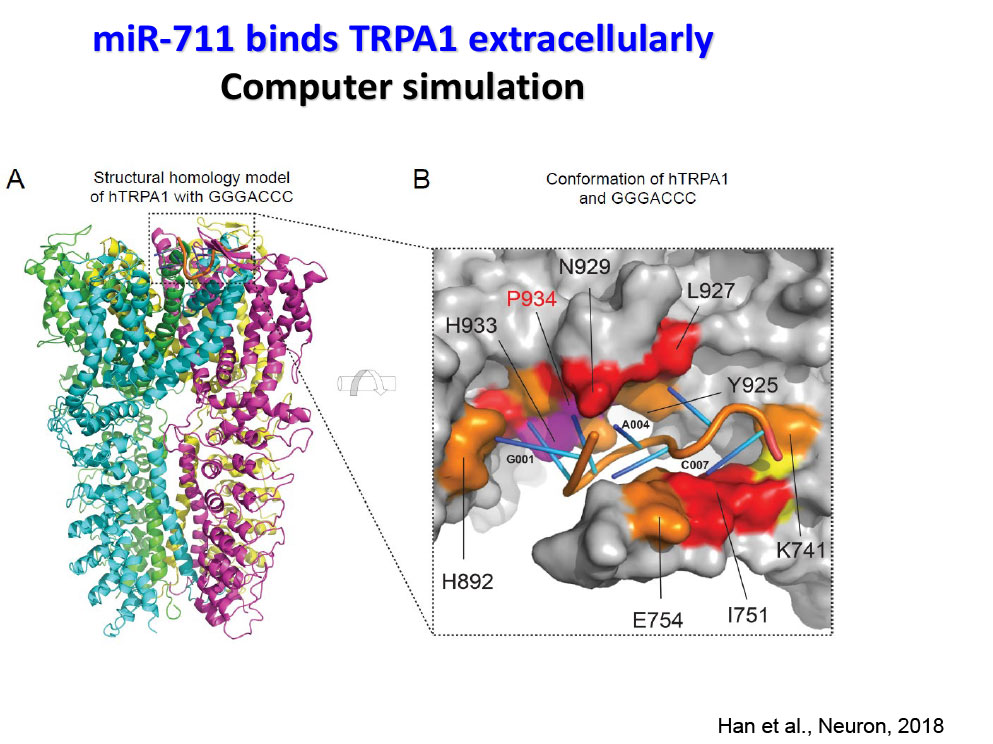
- Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, Luo X, Zhang X, Nackley A, Dokholyan NV, Ji RR. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron. 2018 Aug 8;99(3):449-463.e6.
- Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018 Aug 1;128(8):3568-3582.
- Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018 Aug;129(2):343-366.
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature. 2017 Nov 8;551(7679):192-197.
- Chen G, Kim YH, Li H, Luo H, Liu DL, Zhang ZJ, Lay M, Chang W, Zhang YQ, Ji RR. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat Neurosci. 2017 Jul;20(7):917-926.
- Han Q, Kim YH, Wang X, Liu D, Zhang ZJ, Bey AL, Lay M, Chang W, Berta T, Zhang Y, Jiang YH, Ji RR. SHANK3 deficiency impairs heat hyperalgesia and TRPV1 signaling in primary sensory neurons. Neuron, Dec 21;92(6):1279-1293, 2016
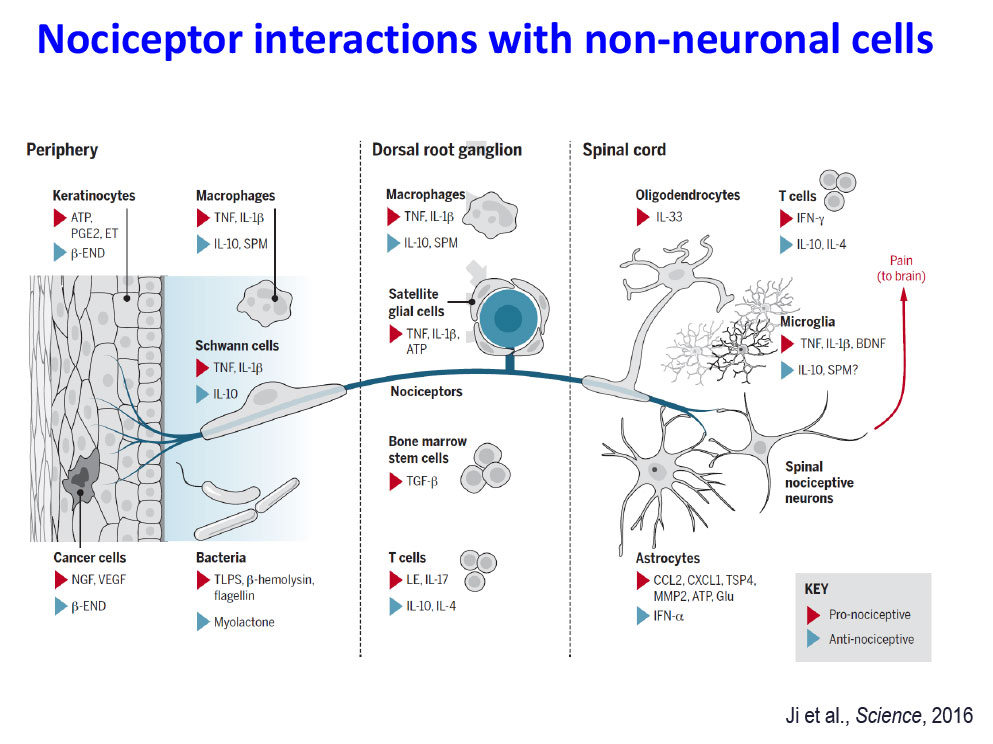
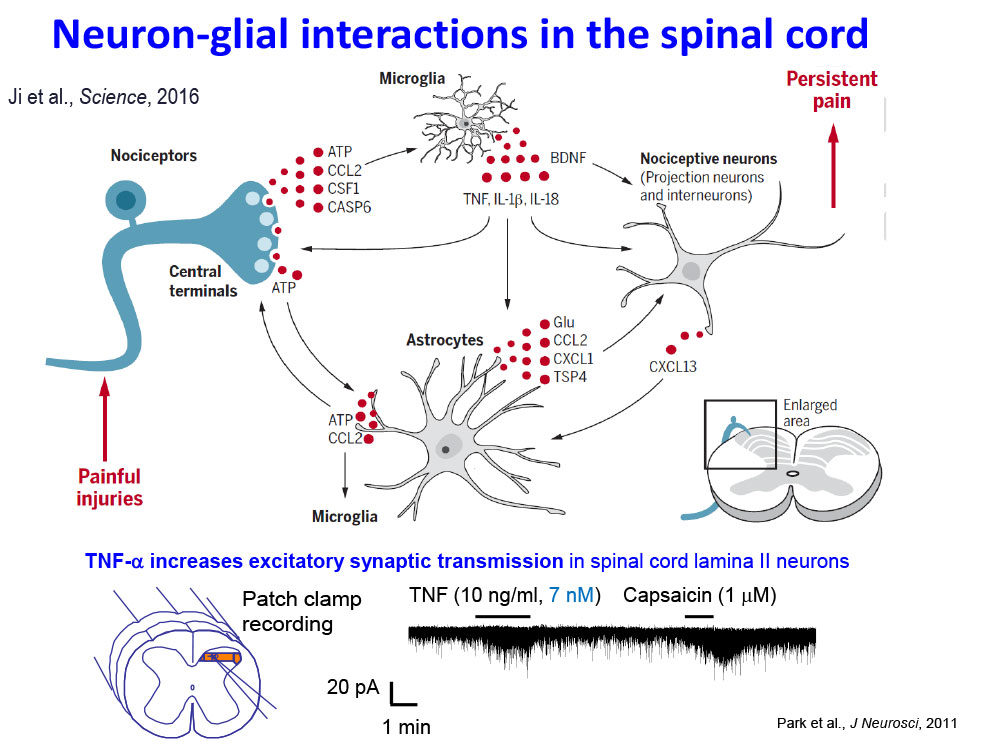
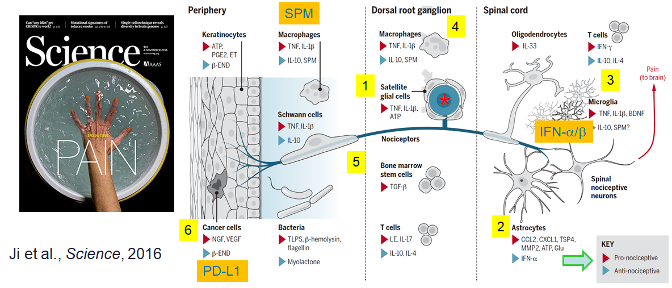
- Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science, 2016, Nov 4; 354(6312):572-577.
- Chen G, Xie RG, Gao YJ, Xu ZZ, Zhao LX, Bang S, Berta T, Park CK, Lay M, Chen W, Ji RR. β-arrestin-2 regulates NMDA receptor function in spinal lamina II neurons and duration of persistent pain. Nat Commun. 2016 Aug 19;7:12531. doi: 10.1038/ncomms12531.
- Jiang BC, Cao DL, Zhang X, Zhang ZJ, He LN, Li CH, Zhang WW, Wu XB, Berta T, Ji RR, Gao YJ. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest. 2016 Feb;126(2):745-61.
- Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, Kim IH, Manhaes AC, Rodrigues WS Jr, Pamukcu A, Enustun E, Ertuz Z, Scheiffele P, Soderling SH, Silver DL, Ji RR, Medina AE, Eroglu C. Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin. Cell 2016 Jan 14;164(1-2):183-96.
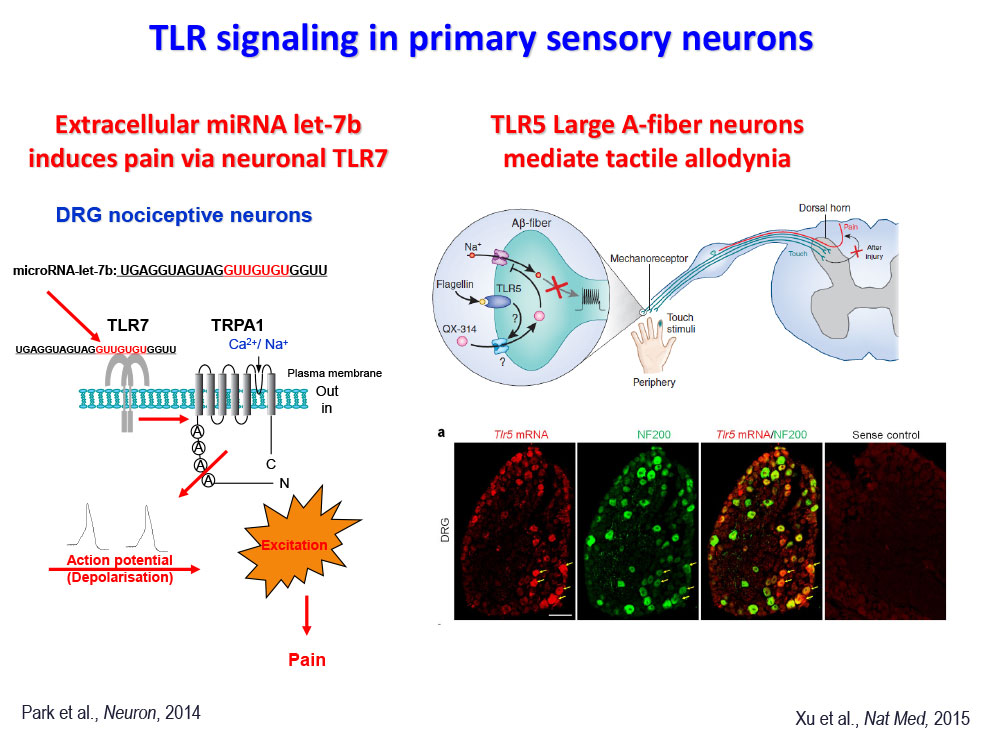
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nature Medicine. 2015 21(11):1326-3.
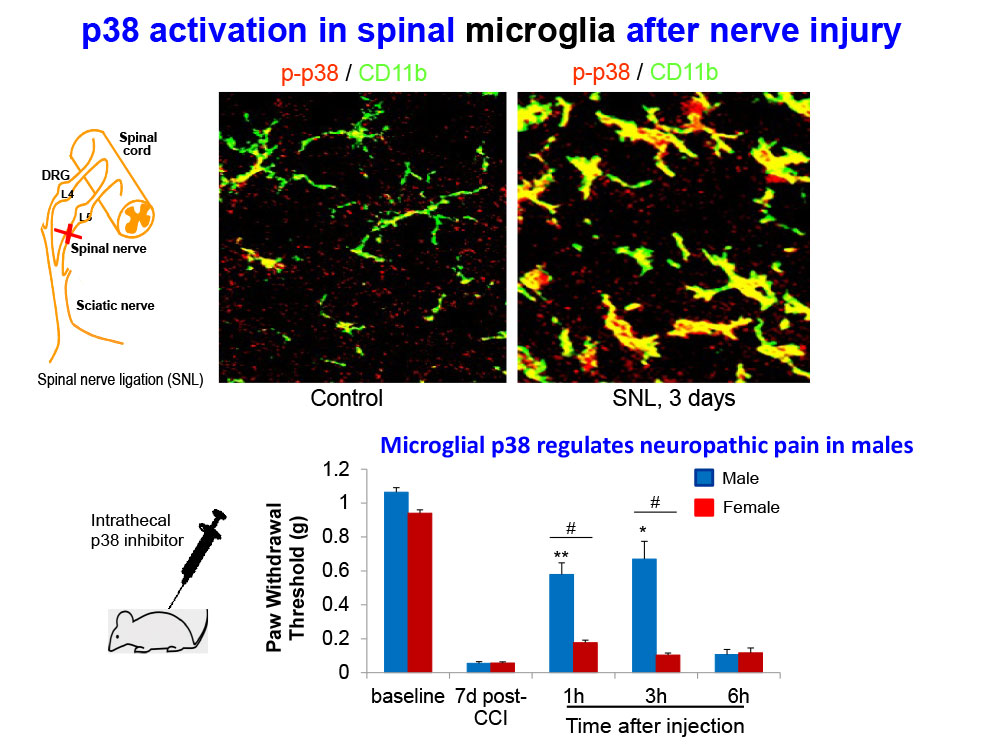
- Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2015 Oct 19. pii: S0889-1591(15)30032-5.
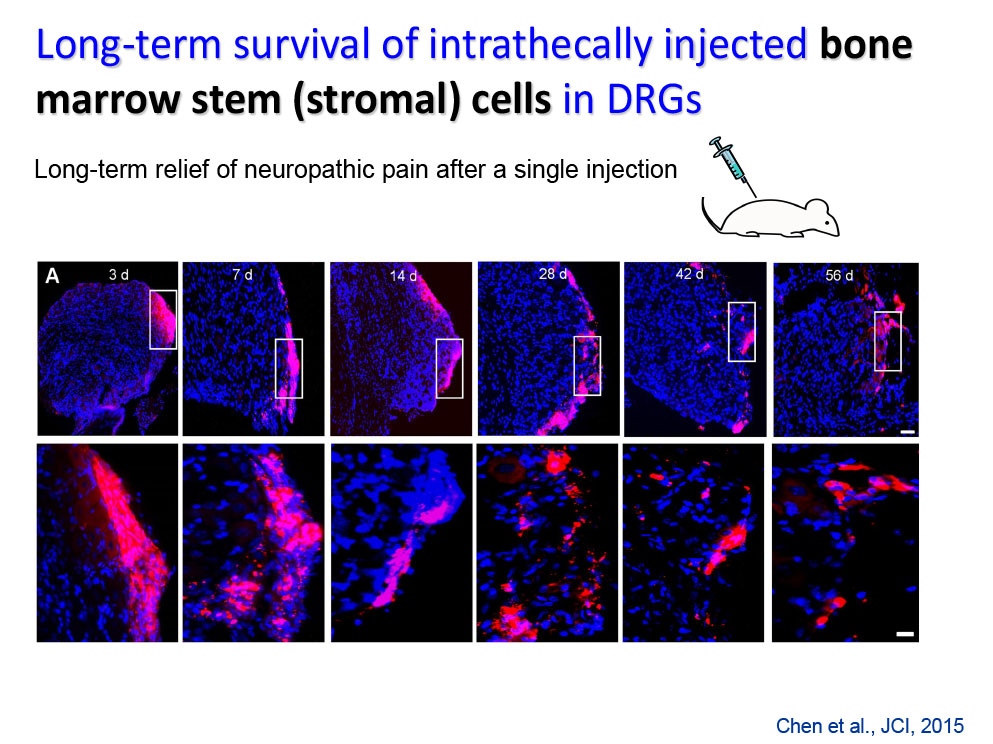
- Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-b secretion. J Clin Invest. 2015;125(8):3226-40.
- Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Michael W Salter MS, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. 2015, Nat Neurosci, 2015 Aug;18(8):1081-3.
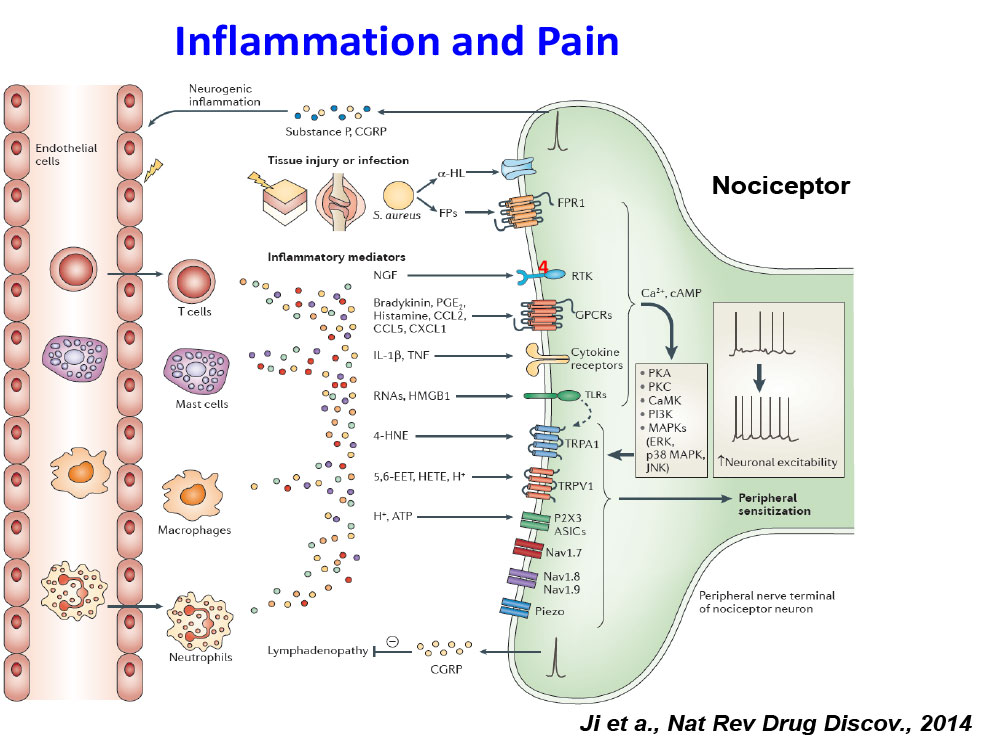
- Ji RR, Xu ZZ, Gao YJ. Neuroinflammation drives chronic pain: emerging targets with pro- and anti-inflammatory roles. Nature Reviews Drug Discovery, 2014, 13:533-548.
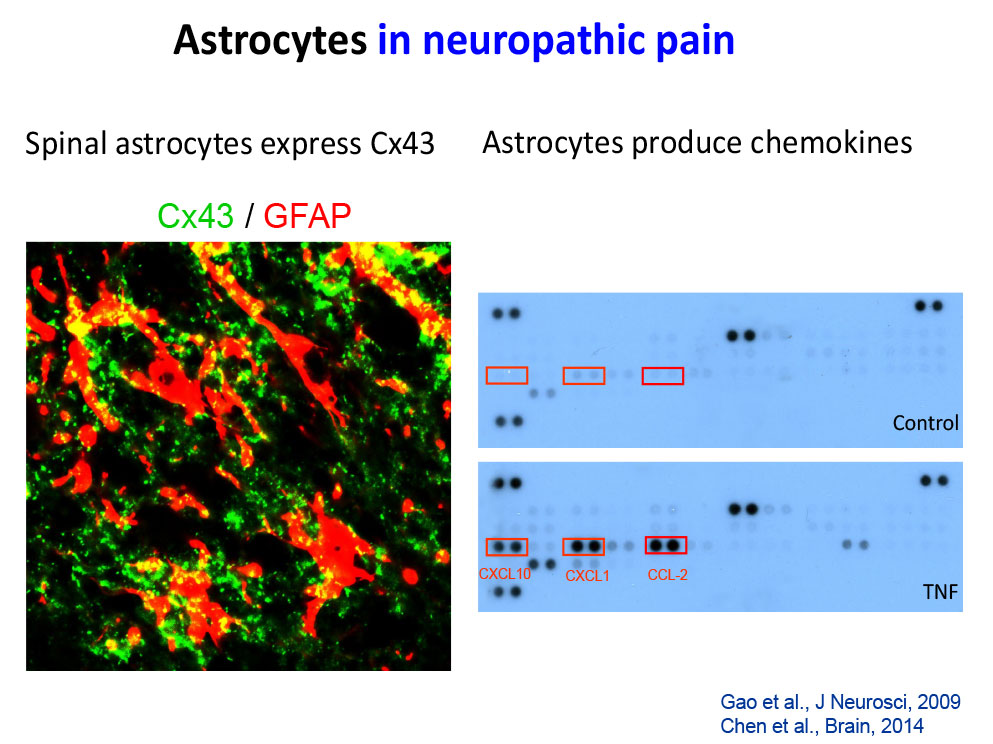
- Chen G, Park CK, Xie RG, Nedergaard M, Ji RR (2014) Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain, 2014, 137:2193-2209.
- Park CK, Xu ZZ, Berta, T, Han QJ, Liu XJ, Ji RR (2014) Extracellular miRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron, 82:47-54.
- Berta T, Park CK, Xie RG, Xu ZZ, Lu N, Ji RR (2014) Extracellular caspase-6 drives murine inflammatory pain via microglia TNF-a secretion. J Clin Invest. 2014; 124:1173-86.
- Ji RR, Berta T, and Nedergaard M. Glia and pain: Is chronic pain and gliopathy? Pain, 2013, 154 Suppl 1: S10-28.
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013, 123:4050-62.
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lü N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012, 122:2195-2207.
For a complete listing of publications in PubMed, click here (or search Ji RR, PubMed).
Lab Techniques Photos
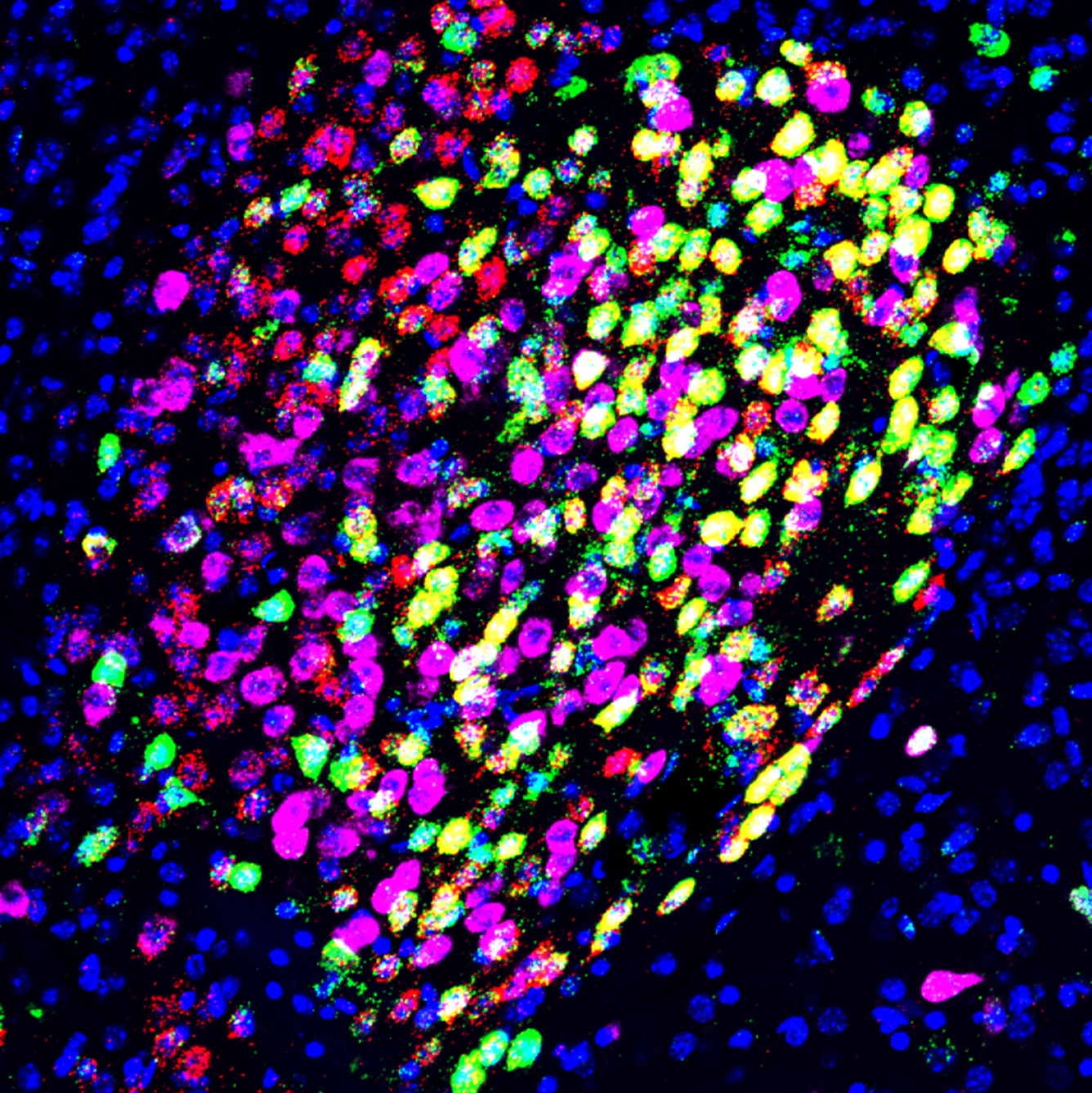
General anesthesia induced neuronal activation in the central amygdala.
Following isoflurane treatment (1.2%, 1.5h), Fos+ (green) neurons are induced in the central amygdala CeA. These general anesthesia (GA) activated neurons (CeAGA) co-express PKCδ (prkcd, red) but not somatostatin (Sst, violet). All the cell nuclei in the image are labeled with DAPI (blue).
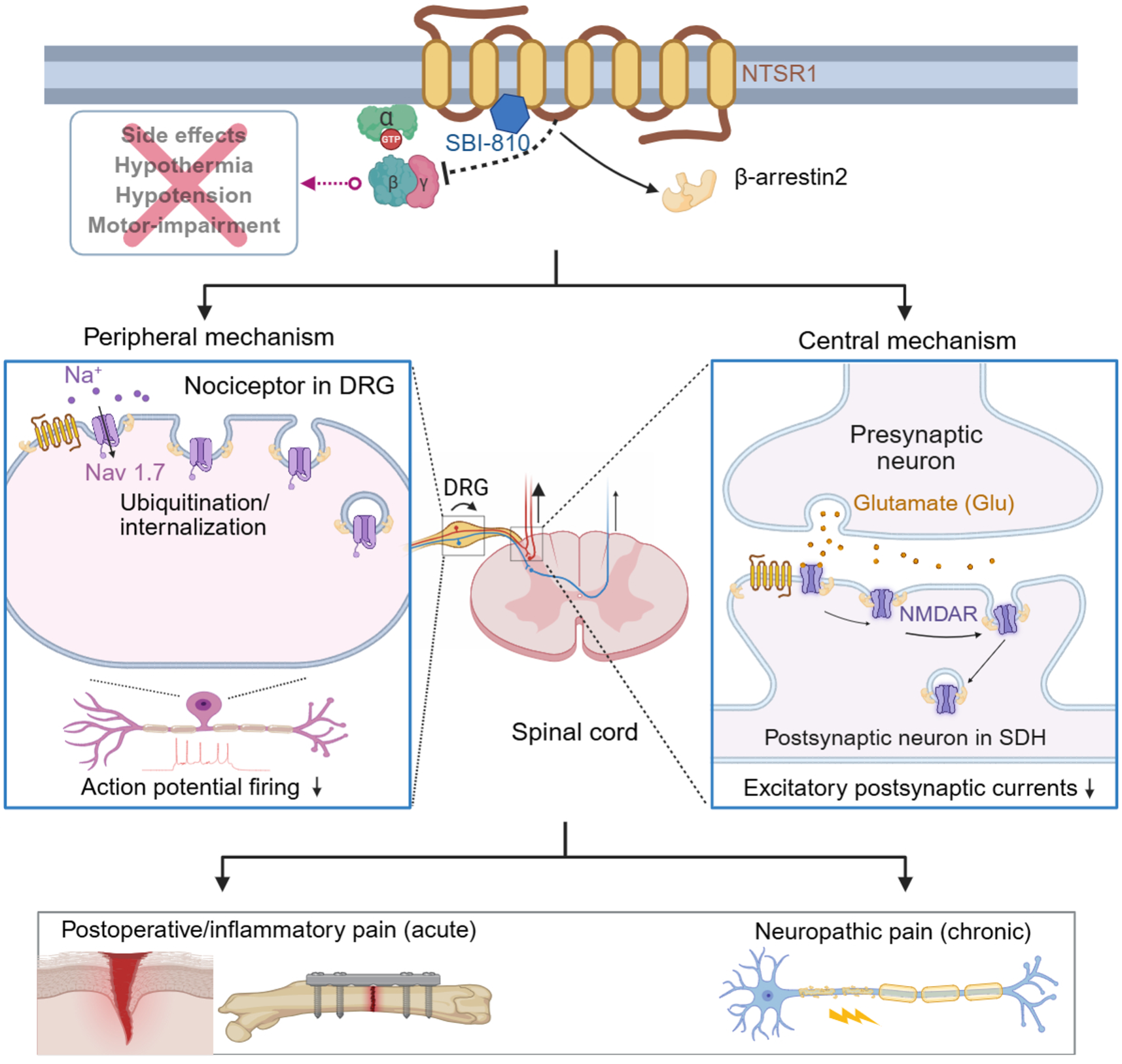
Arrestin-biased allosteric modulator of neurotensin receptor 1 alleviates acute and chronic pain. The allosteric modulator SBI-810 inhibits pain via both peripheral and central modulation.