January 2014
History
The patient is a 69 year old female with significant past medical history of hypertension, hyperlipidemia, type2 diabetes mellitus, obesity, and atrial fibrillation. She presents to clinic with an episode of chest discomfort (resolved spontaneously) and new onset murmur discovered by PCP. She denies dyspnea, syncope, or signs and symptoms of heart failure. She denies fevers, night sweats, or any previous cardiac surgery.
Echo (TTE)
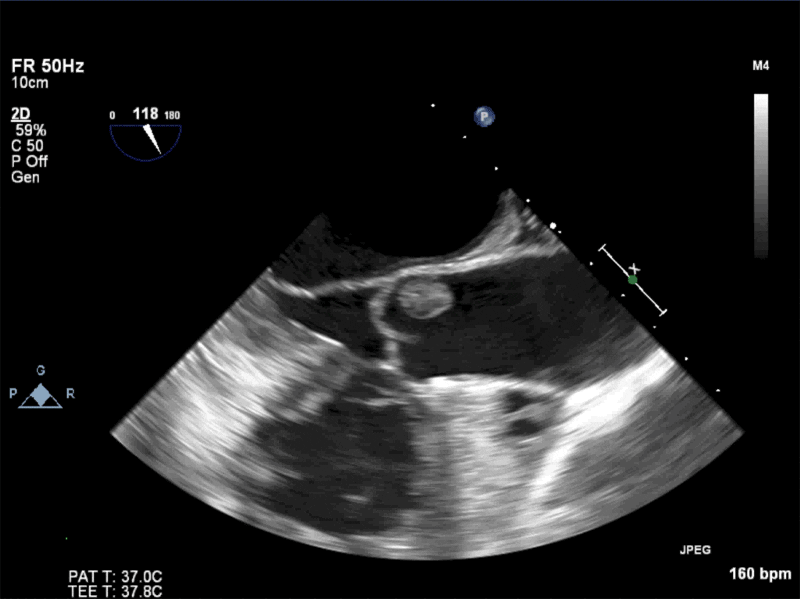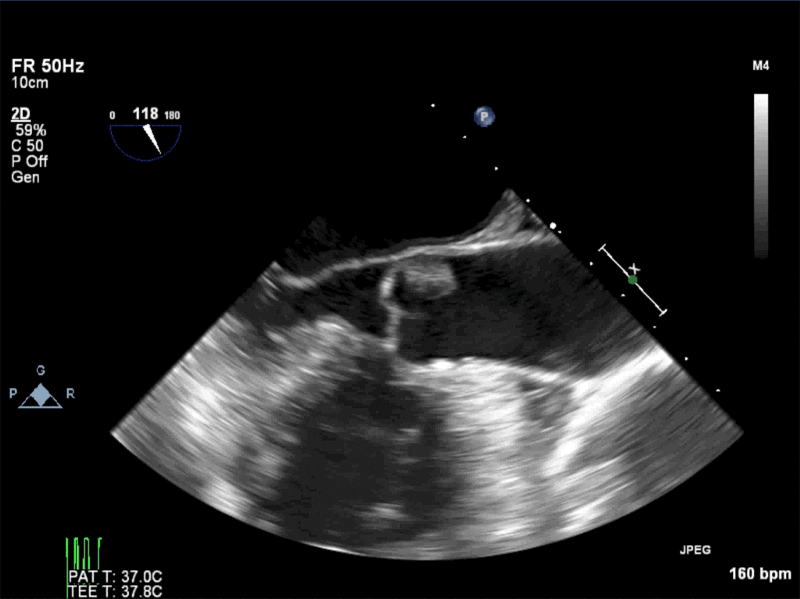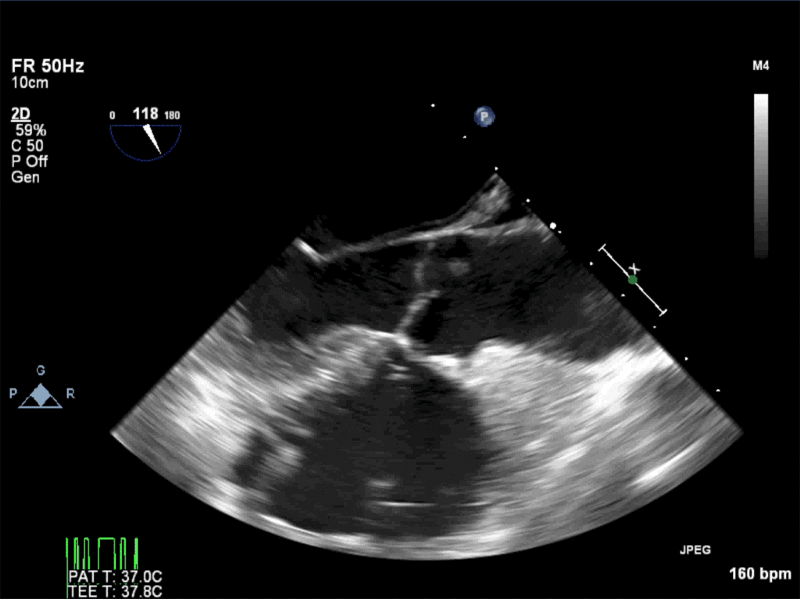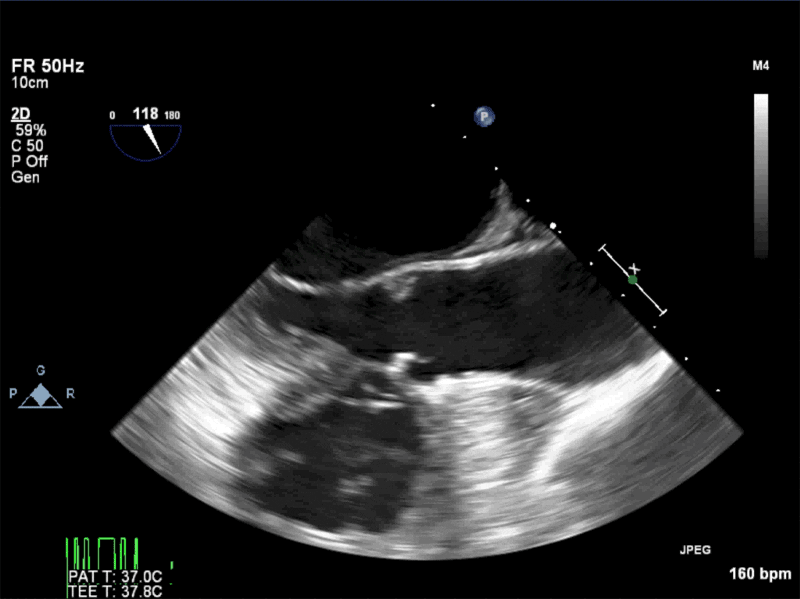
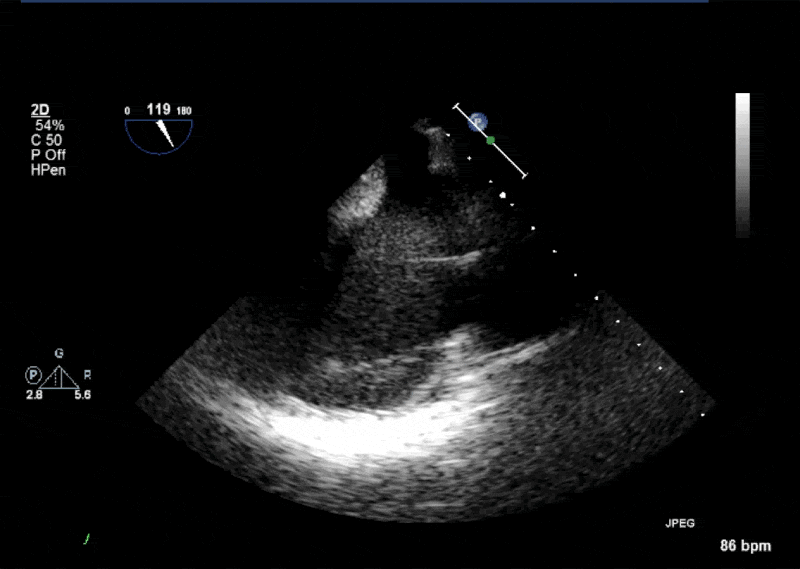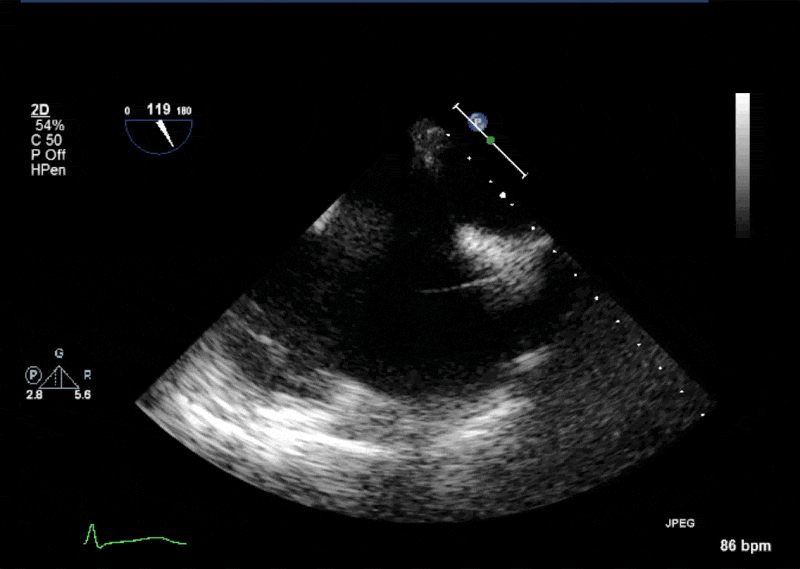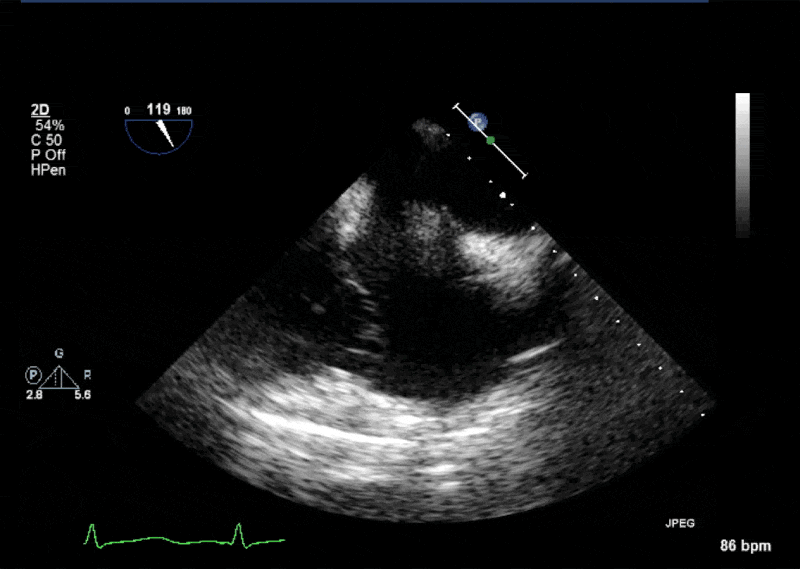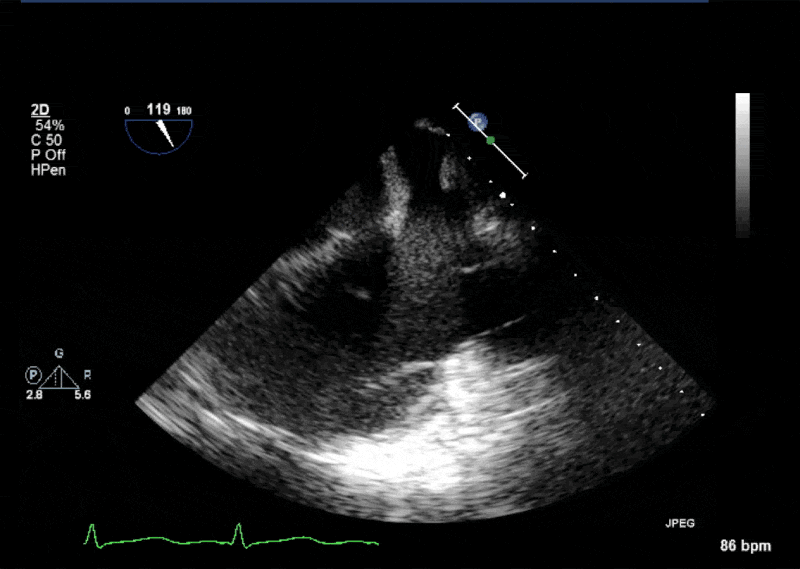
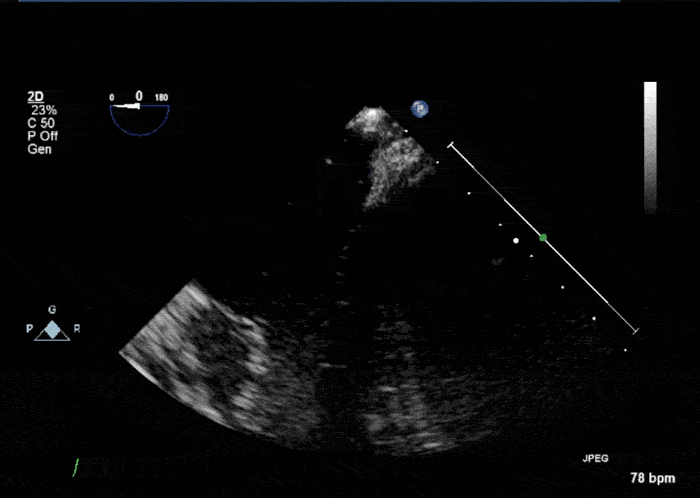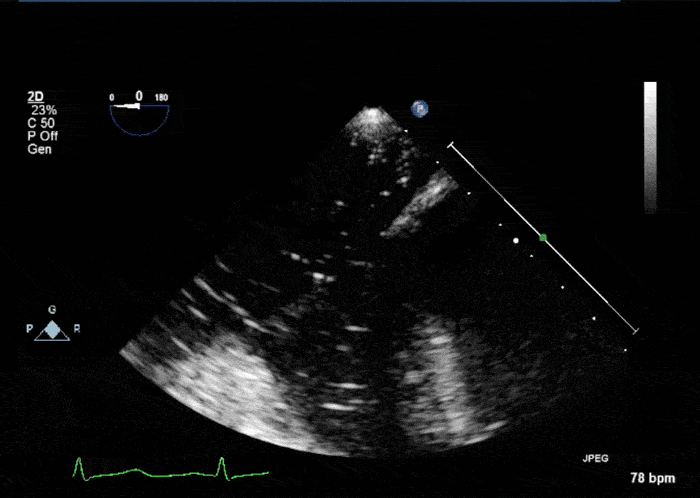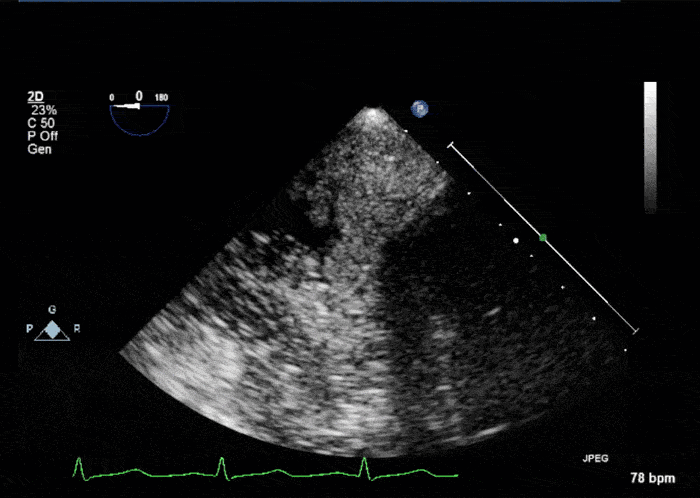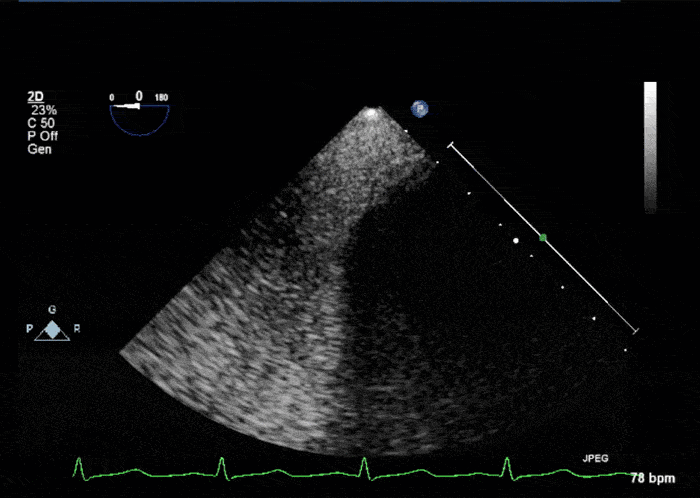
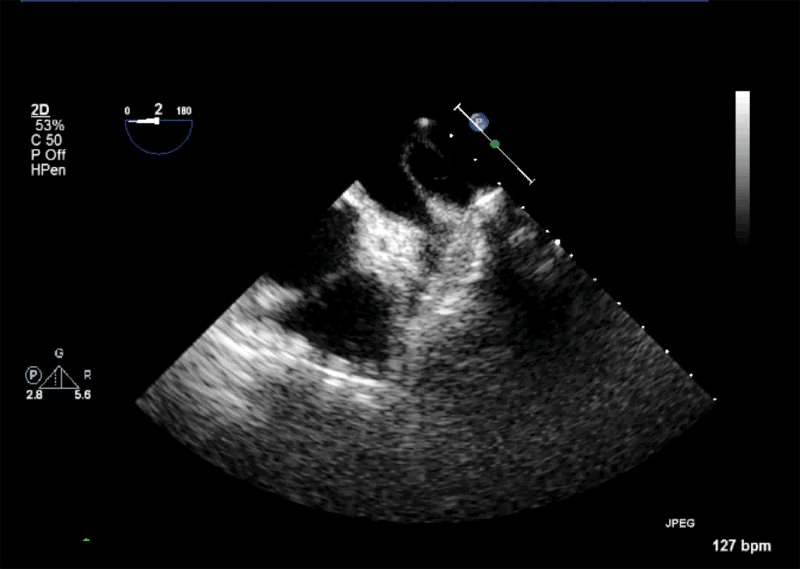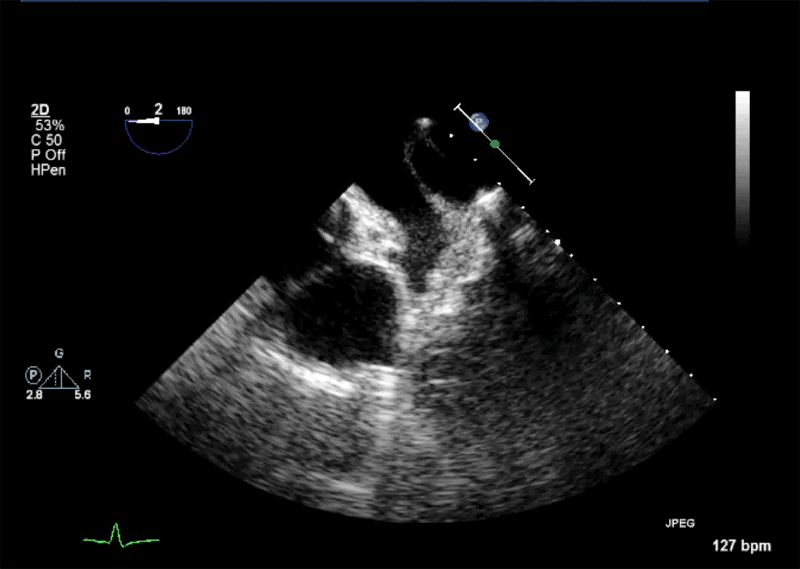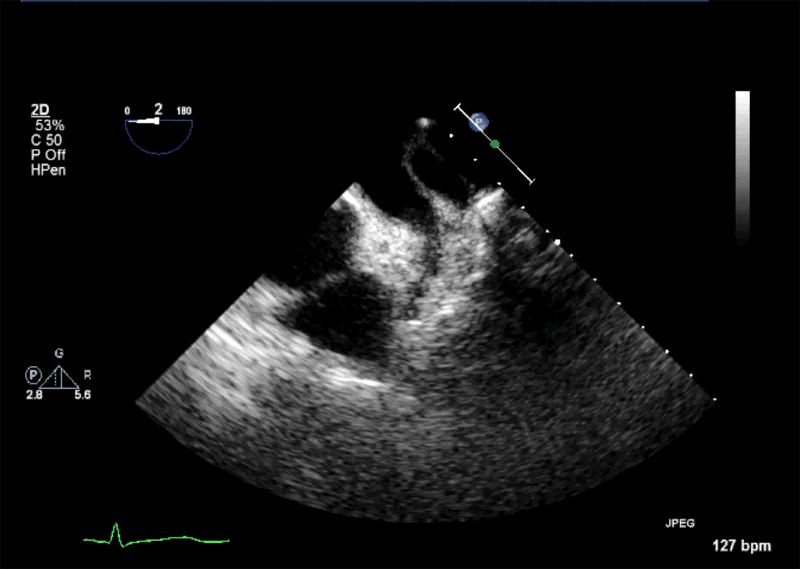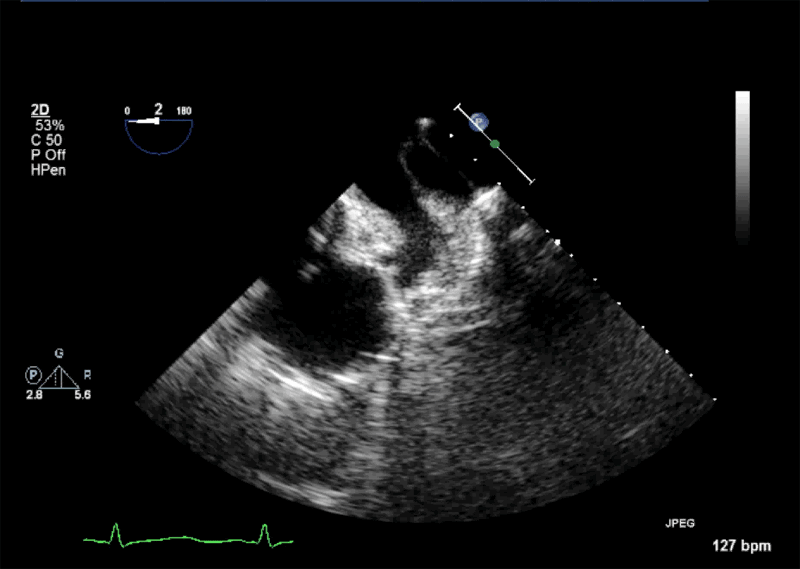
- Echogenic mass (0.9 cm x 1.1 cm) noted on aortic side of the aortic valve
- Precise mass attachment point uncertain – non-coronary cups of the aortic valve vs. proximal aortic wall
Differential
| Myxoma | Papillary Fibroelastoma | Lipoma | Thrombus | Endocarditis | Lambl’s | |
|---|---|---|---|---|---|---|
| Incidence | Up to 50% intra-cardiac tumors | Up to 8% intra-cardiac tumors | 2nd most common intra-cardiac | Common with Prosthetic Valves (mech >>biop)1 LVAD, or ECMO | More common prosthetic valve | 40% |
| Location | IAS attachment | Valvular endocardium | Anywhere Valve location is RARE2 | Uncommon on valve unless above conditions | Left-sided lesions 90% of cases | Aortic and/or ventricular surface of aortic valve |
| Echo | Heterogeneous Broad based attachment | Homogenous with stalk3 | Homogenous, Encapsulated | No clear point of attachment Echogenicity different than myocardium | Move in phase with valves since | History important in differentiating from endocarditis and fibroelastoma |
| Addtnl | Frequent presentation: dyspnea, chest pain, fatigue.4 | 75% of valvular tumors are papillary fibroelastomas.5 | Case report of man with protein C deficiency as presumptive cause of Aortic Thrombus.6 | Clinical context important in diagnosis | Thought to be associated with stroke |
Video 1: AV LAX showing mobile mass attached to the left or non-coronary cusp
Video 2: AV SAX showing mobile mass attached to the non-coronary cusp
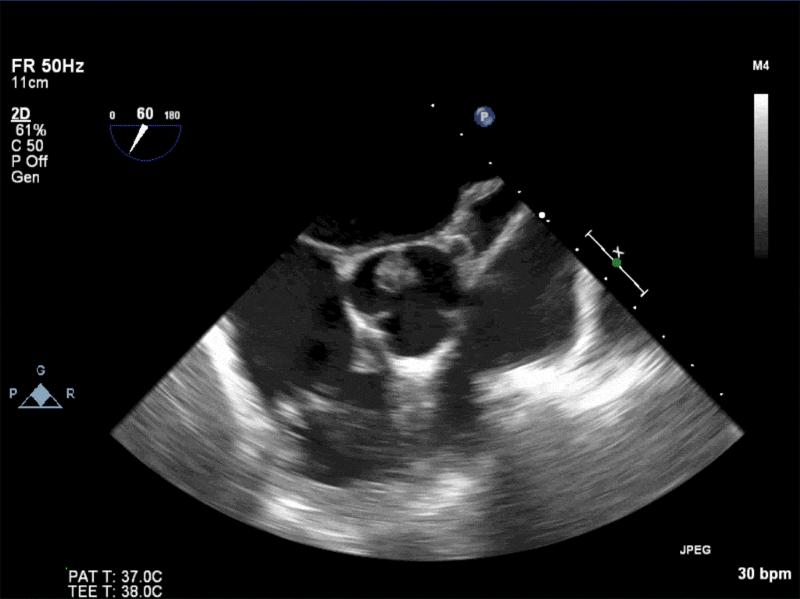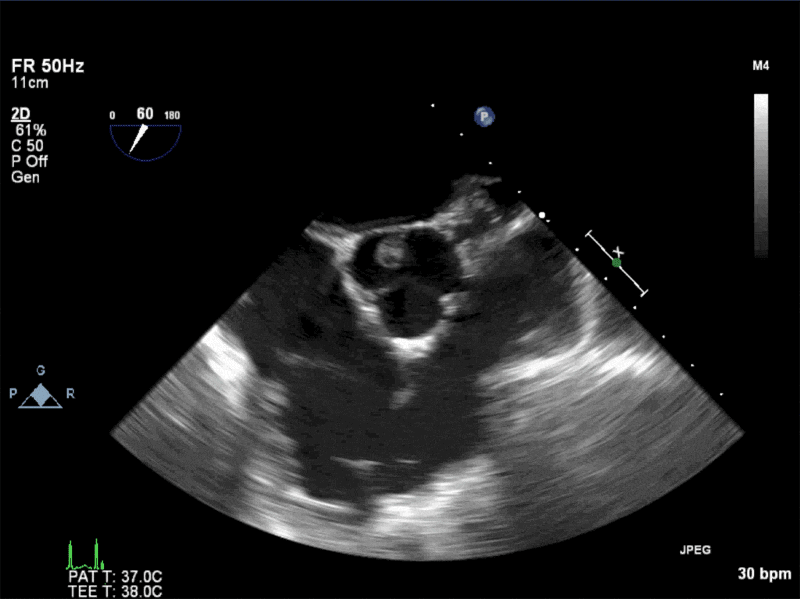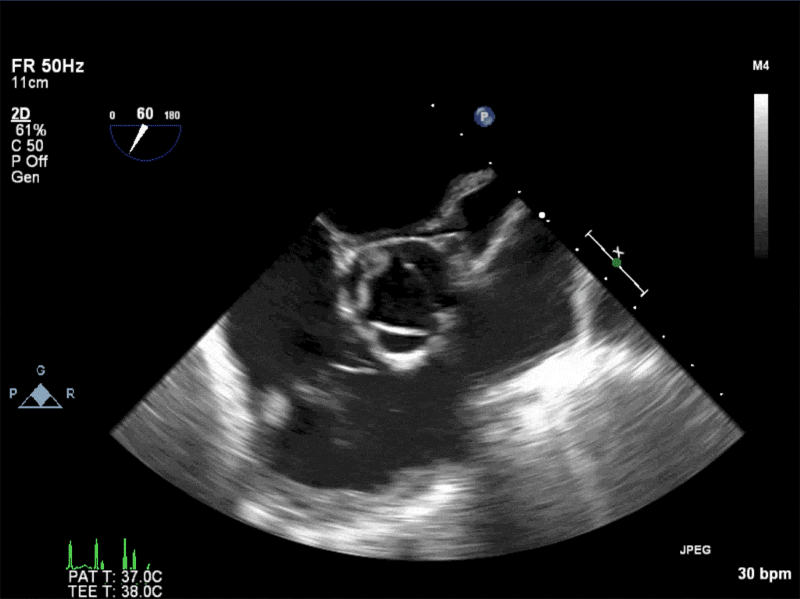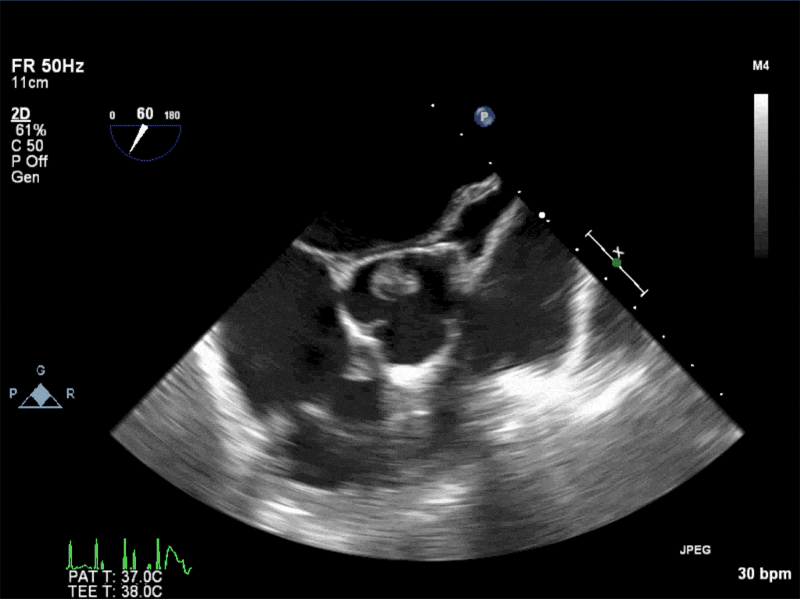
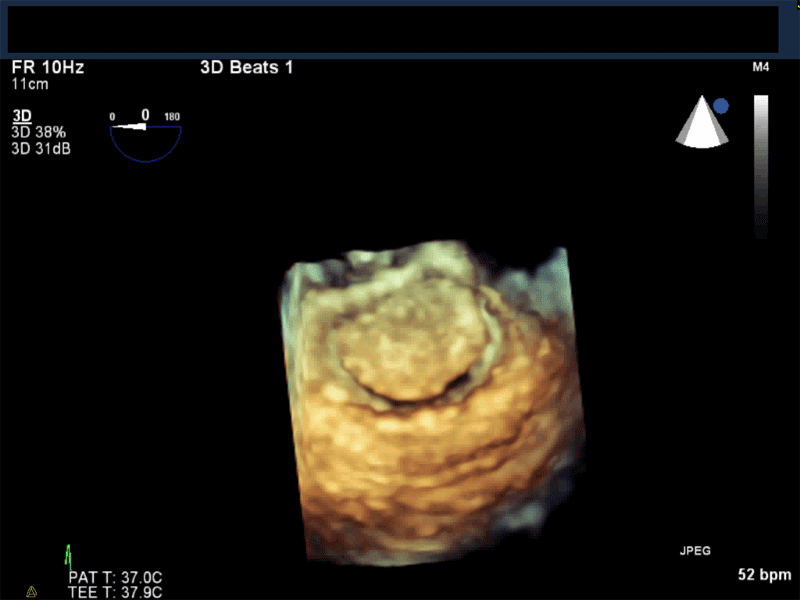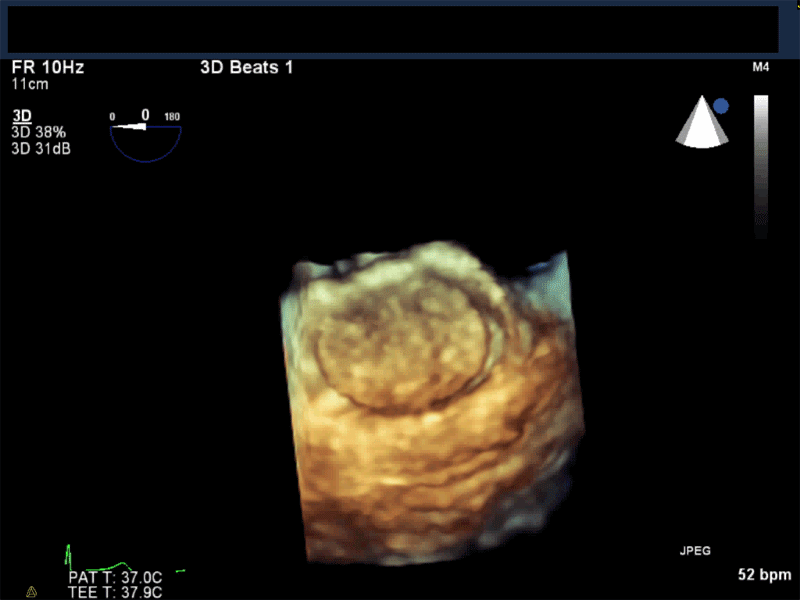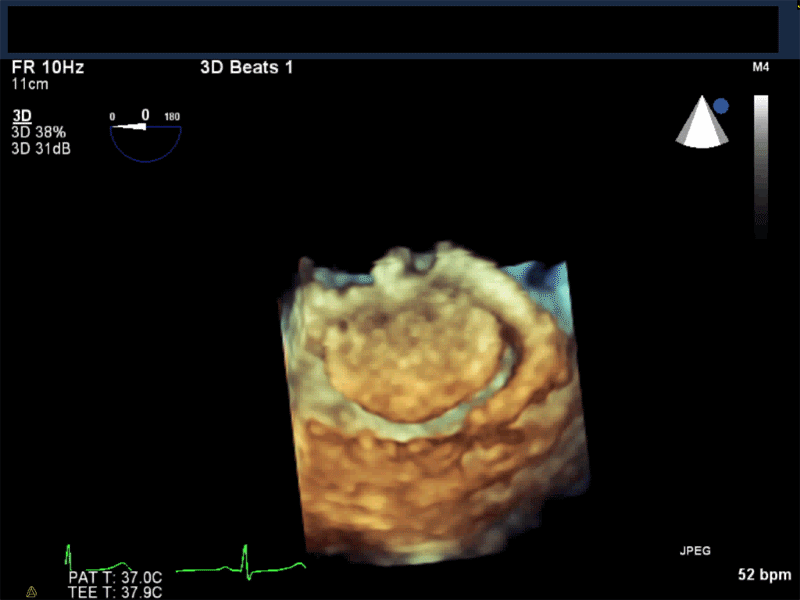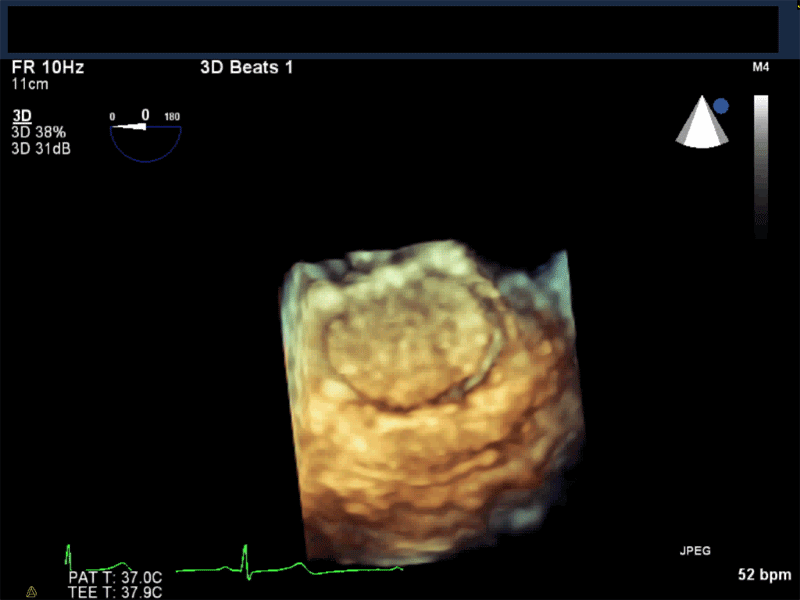
Video 3: AV Short Axis – 3D view of the aortic valve from the ascending aorta showing mobile mass attached to the non-coronary cusp
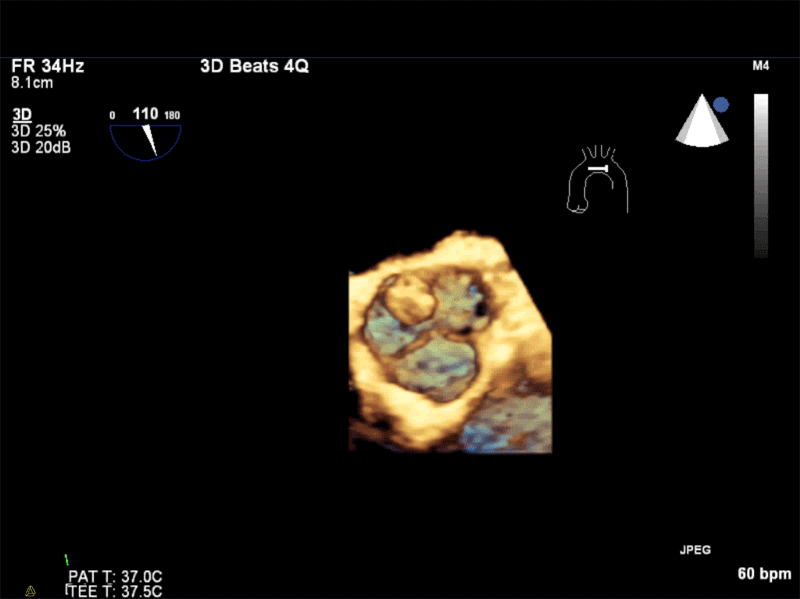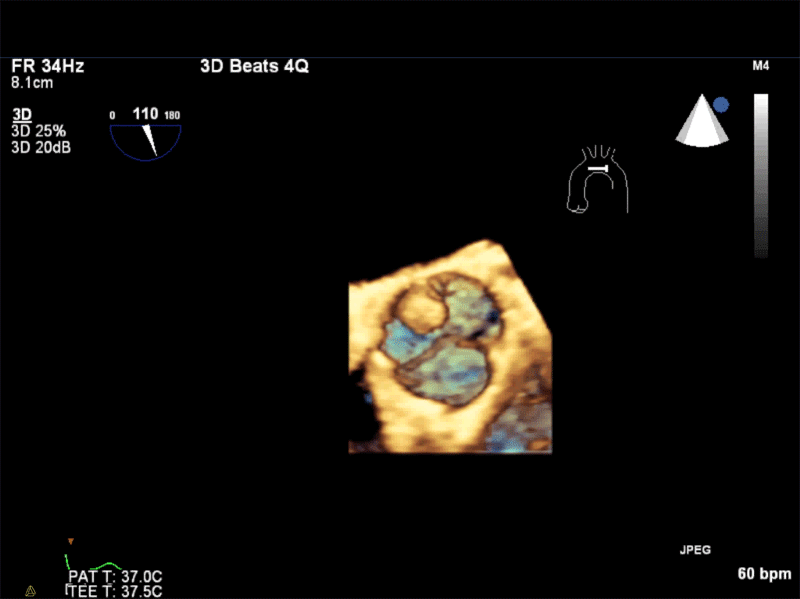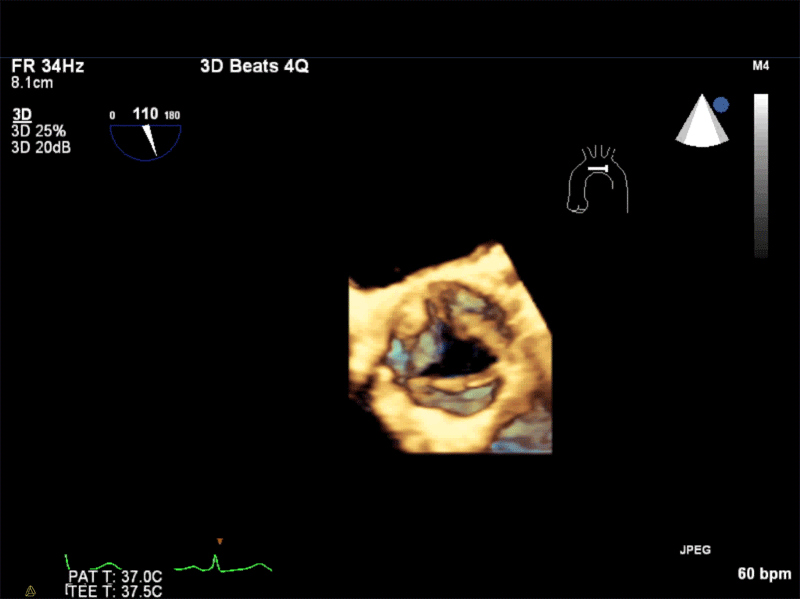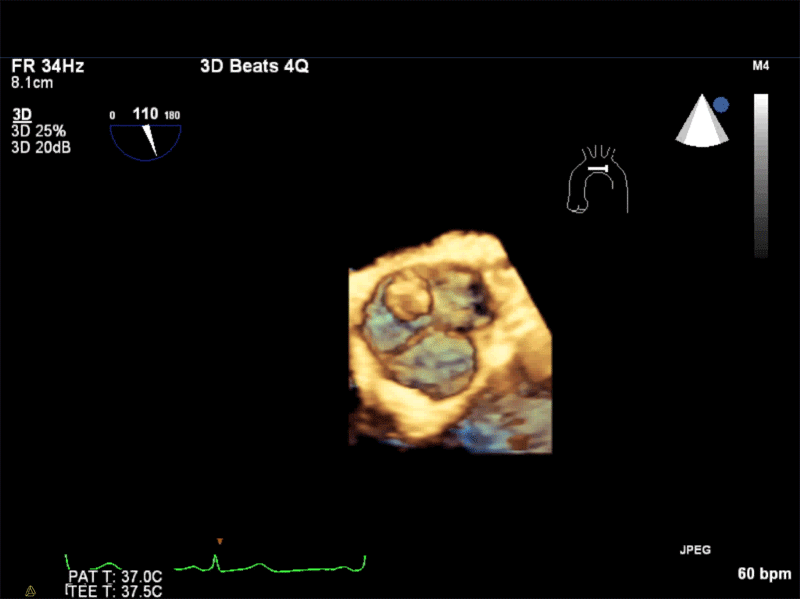
Video 4: AV Short Axis – 3D Cropped showing attachment point of the mobile mass at the base of the non-coronary cusp
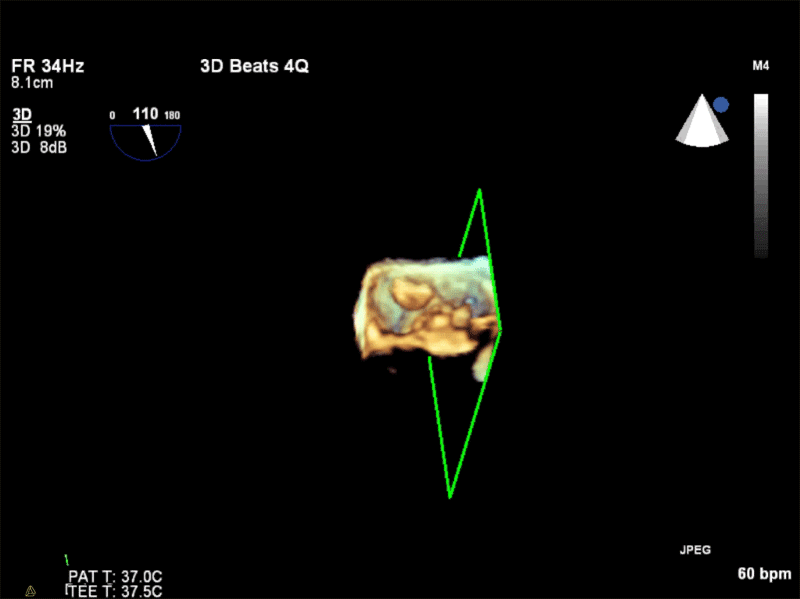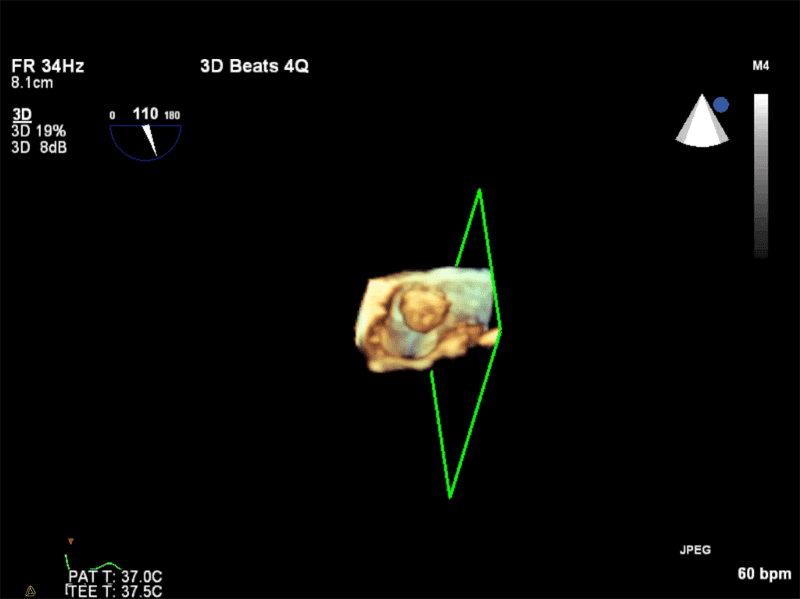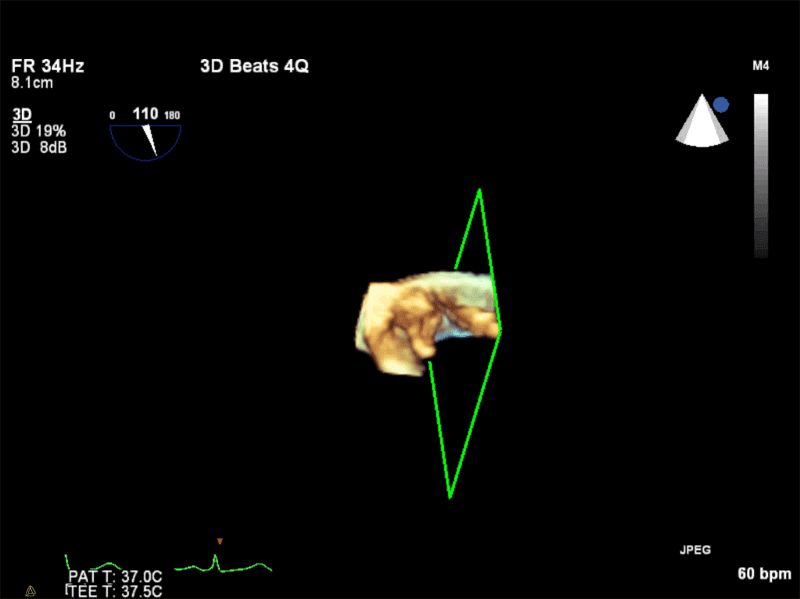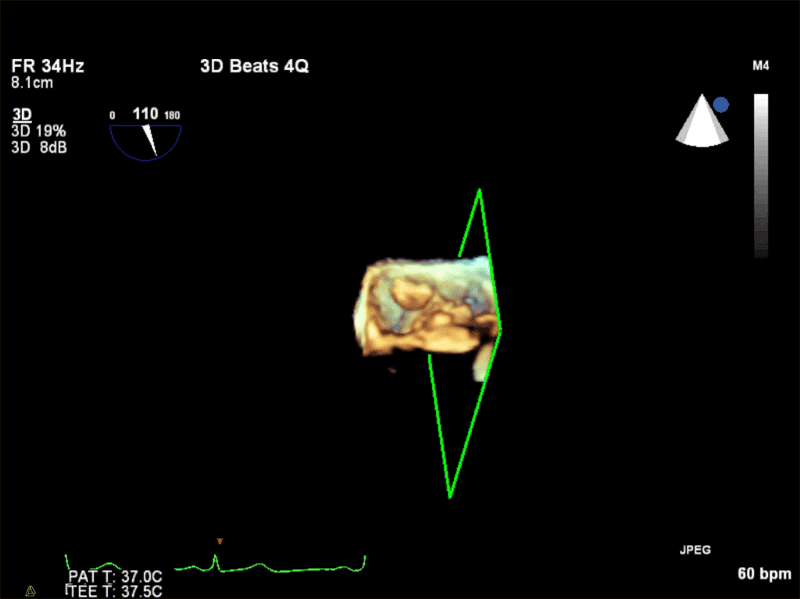
Pathology
1.1 x 0.9 x 0.5 cm tan, gelatinous soft portion of tissue
Diagnosis Papillary Fibroelastoma
References
- Ohnaka M, Nishimura K, Kurokawa S. Flat fibrin thrombus deposition on tissue valve after aortic valve replacement. The Annals of thoracic surgery. Jun 2010;89(6):2032-2034.
- Matsushita T, Huynh AT, Singh T, et al. Aortic valve lipoma. The Annals of thoracic surgery. Jun 2007;83(6):2220-2222.
- Seto T, Takano T, Otsu Y, et al. Cardiac Papillary Fibroelastoma: Report of Three Cases. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. Oct 3 2013.
- Burnside N, MacGowan SW. Malignant primary cardiac tumours. Interactive cardiovascular and thoracic surgery. Dec 2012;15(6):1004-1006.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. The Annals of thoracic surgery. Nov 1991;52(5):1127-1131.
- Yokoyama Y, Satoh H, Kurata A, et al. [Surgical removal of native aortic valve thrombosis associated with acute myocardial infarction and protein C deficiency; report of a case]. Kyobu geka. The Japanese journal of thoracic surgery. Mar 2009;62(3):238-240.
January 2014
Persistent left superior vena cava (PLSVC):
- Most common variation in the thoracic venous system
- Present in 0.5% of the population. Can be associated with other congenital anomalies such as: bicuspid aortic valve, atrial septal defect, or coarctation of the aorta
- Can be concomitant with absence of right superior vena cava (SVC) in which case the right internal jugular vein and the right subclavian vein empty through an innominate vein in the PLSVC.
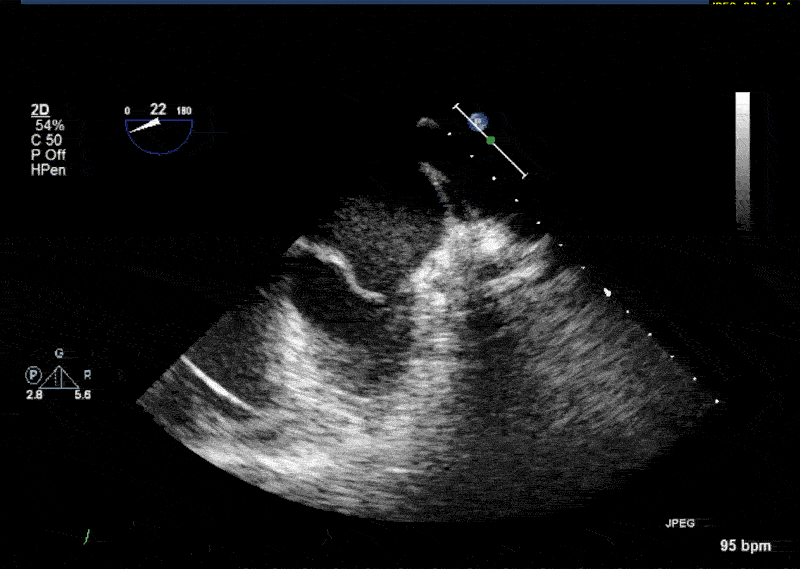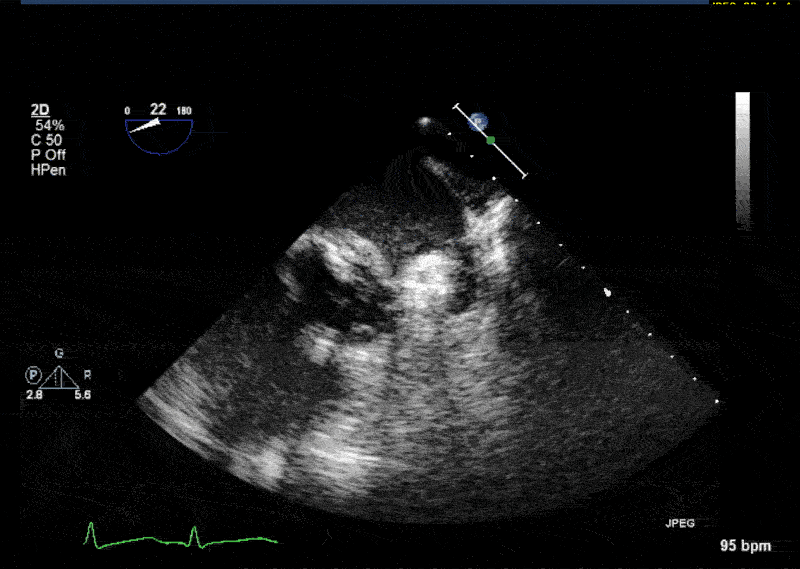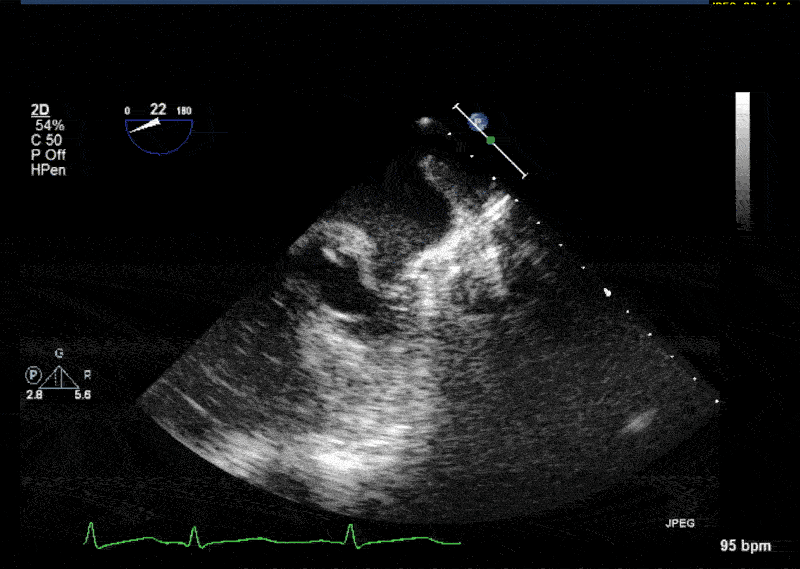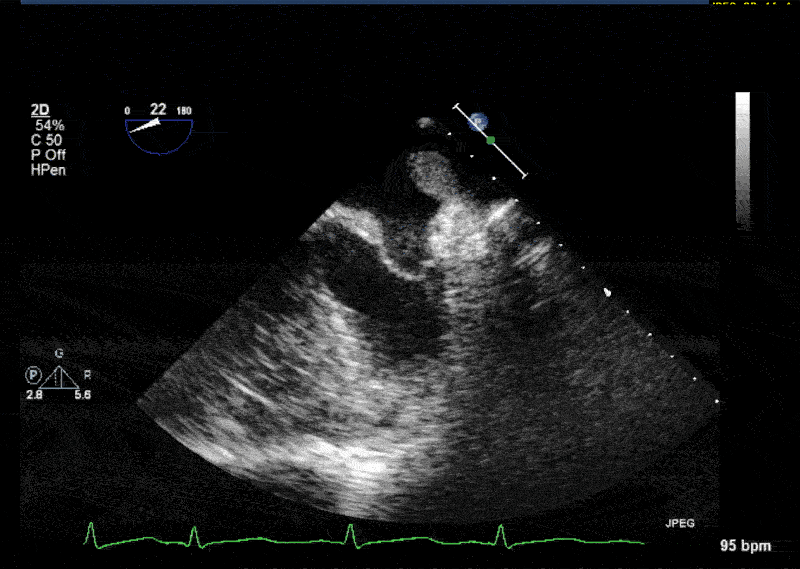
- In 10% of the cases PLSVC empties directly into the left atrium resulting in a small left-to-right shunt. • In most cases PLSVC empties into the right atrium through the coronary sinus. In this situation, PLSVC is associated with a dilated coronary sinus, diameter greater than 1 cm (Video 1 shows a dilated coronary sinus in a modified midesophageal bicaval view). Injection of agitated saline into a venous line situated in the left superior extremity or left neck will result in opacification of the coronary sinus before the opacification of the right atrium (Video 2).
- Normally, the left innominate vein travels laterally to the aortic arch in a parallel fashion. The PLSVC travels laterally to the aortic arch at an orthogonal angle (Video 3 shows an X plane view of the aortic arch; note the dissection flap in the aortic arch and the PLSVC in short axis at 0° and in long axis at 90° adjacent to the aortic arch).
- The PLSVC course then travels between the left atrial appendage and the left upper pulmonary vein (Video 4 and Video 5 show an echo lucent space with agitated saline (Video 5) between the left atrial appendage and the left upper pulmonary vein where the ligament of Marshall—which is the fibrosed left SVC—should be)
- The presence of PLSVC has several implications in (1) placement of a central line, (2) floating of a pulmonary catheter, (3) administration of retrograde cardioplegia , and (4) venous drainage during cardiac surgical procedures on the right heart.
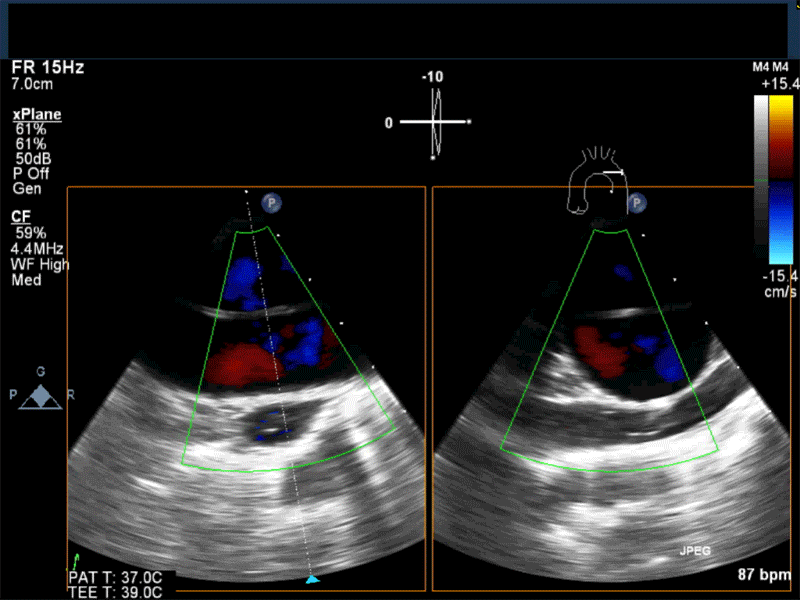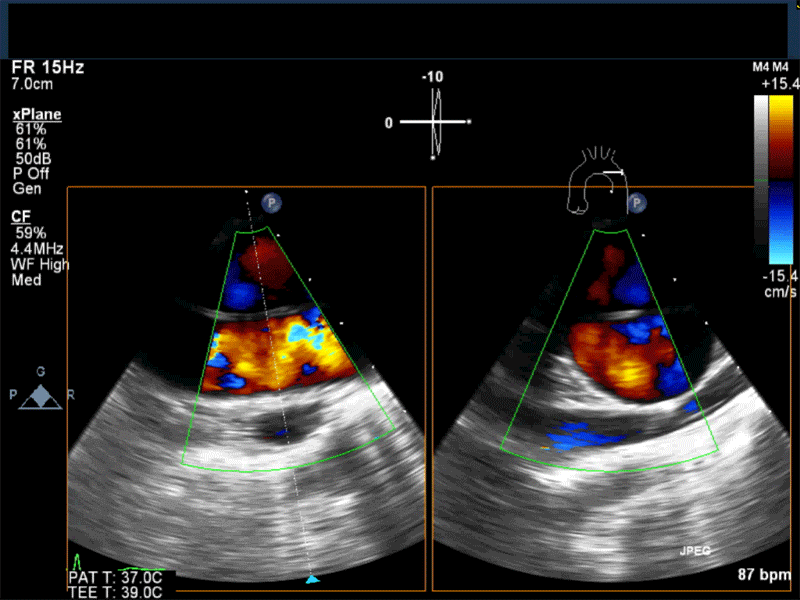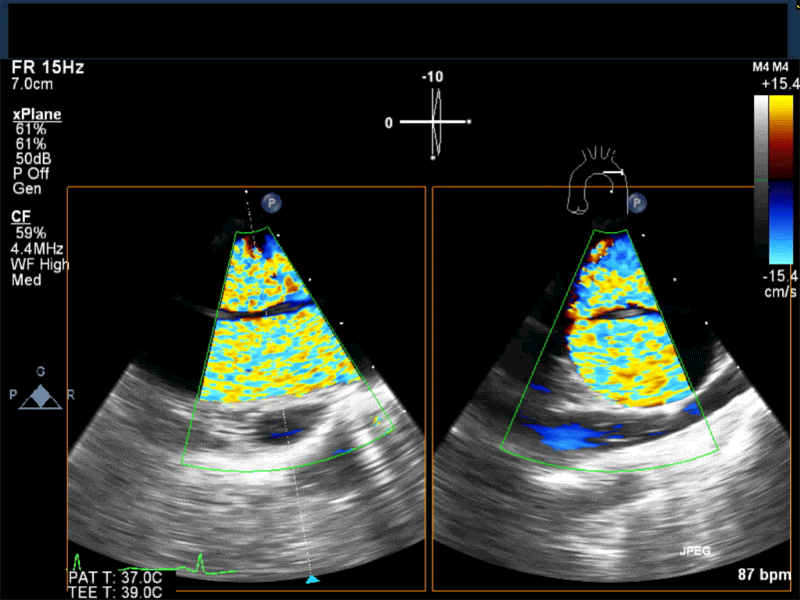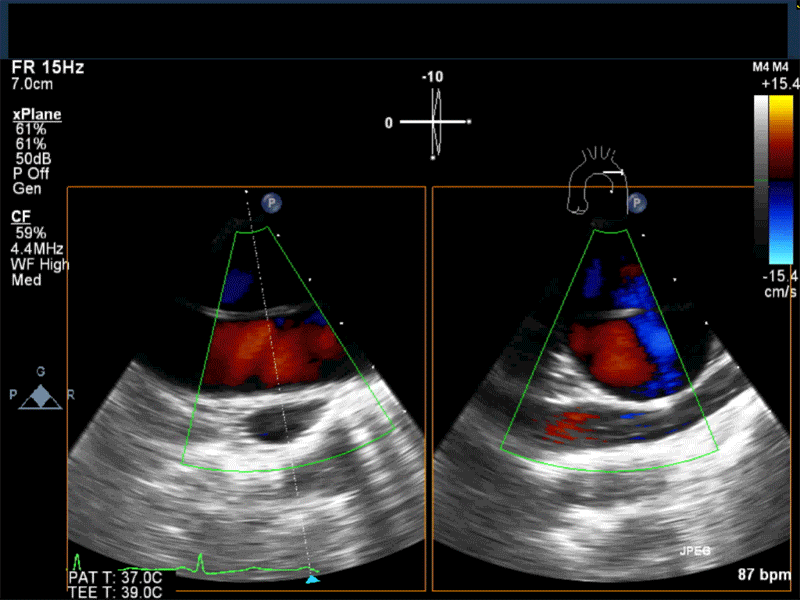
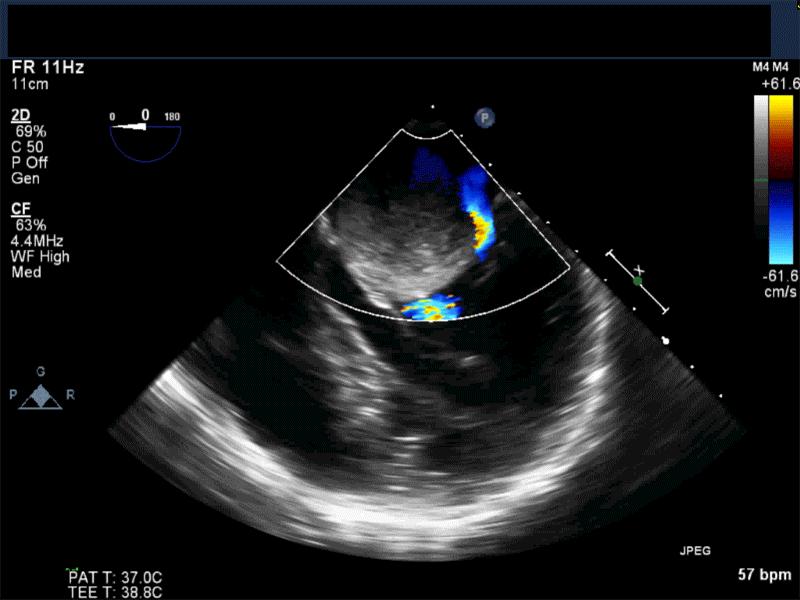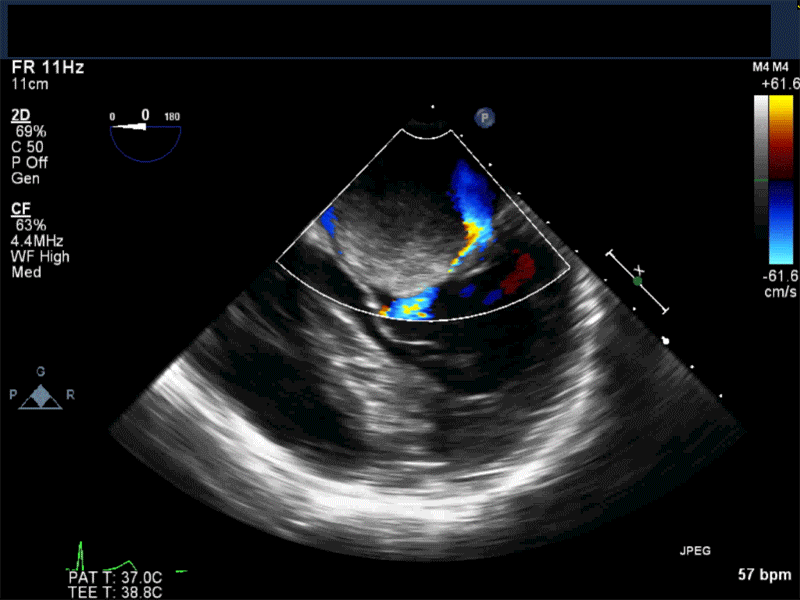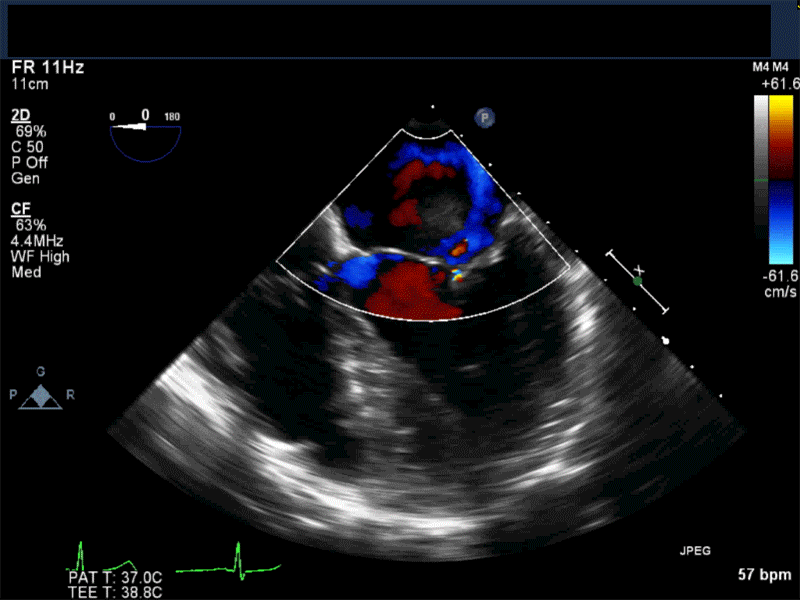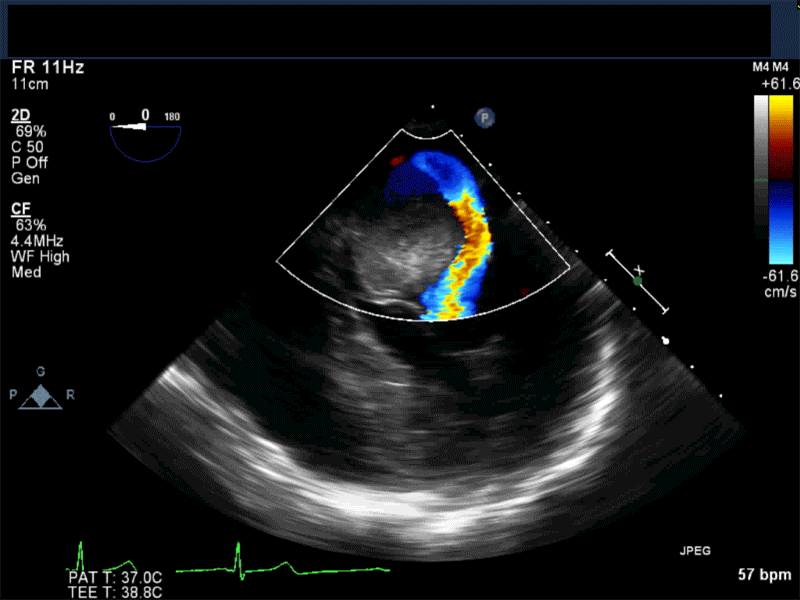
- Myxomas are the most common primary neoplasm of the heart, with an incidence of 0.5 per million population per year, and they account for 50% of all primary cardiac tumors diagnosed at the time of autopsy. Myxomas are most commonly diagnosed in the 3rd-6th decade and have a 65% female predominance. Up to 10% of myxomas are associated with familial syndromes such as Carney complex.
- Patients may be asymptomatic, or may present with constitutional, embolic or obstructive symptoms. Approximately 75-85% of myxomas occur in the left atrium, 5-20% occur in the right atrium, and a small percentage are found in the ventricles or in multiple locations. On TEE, myxomas usually appear as sessile or pedunculated heterogeneous structures and are most commonly attached by a stalk to the interatrial septum in the region of the fossa ovalis. Mxyomas may prolapse through the atrioventricular valve and can cause obstruction or damage to the valve.
- The pre-procedure TEE exam should focus on classifying the tumor and attachment site (using 3-D if necessary), evaluating the associated valve for functional stenosis or valvular damage and ensuring multiple myxomas are not present. The post-CPB exam should focus on ensuring a complete resection has taken place and verifying that no residual septal defect or valvular insufficiency is present.
Video 1 Midesophageal 4-chamber view with color flow Doppler shows large myxoma prolapsing through and obstructing the mitral valve leading to functional mitral valve stenosis.
Video 2 Midesophageal long axis view with color flow Doppler
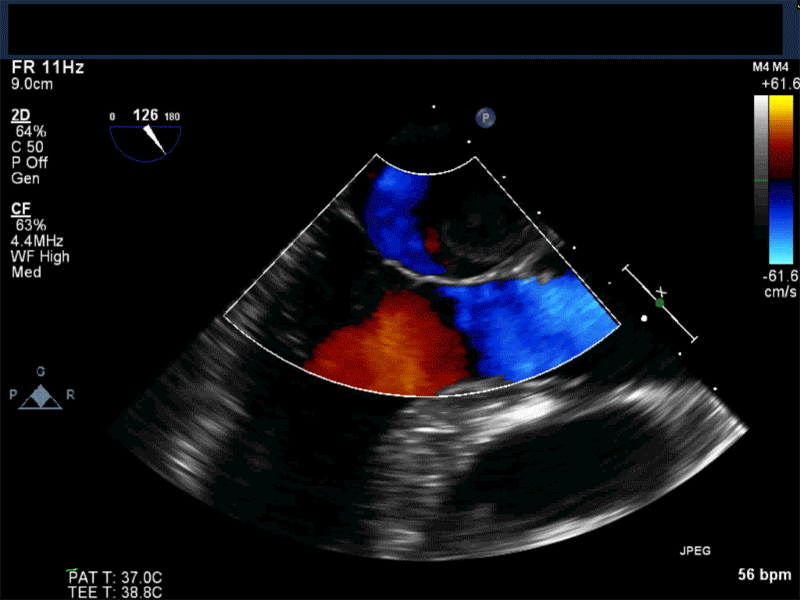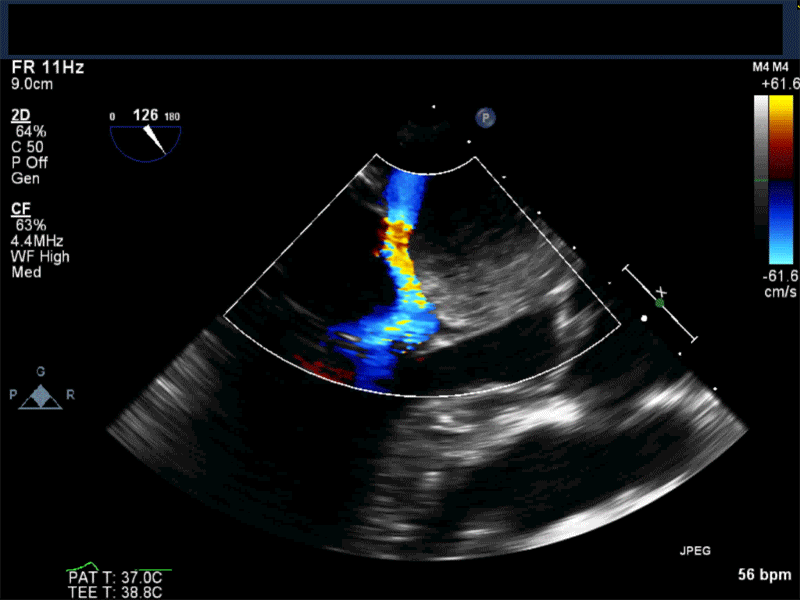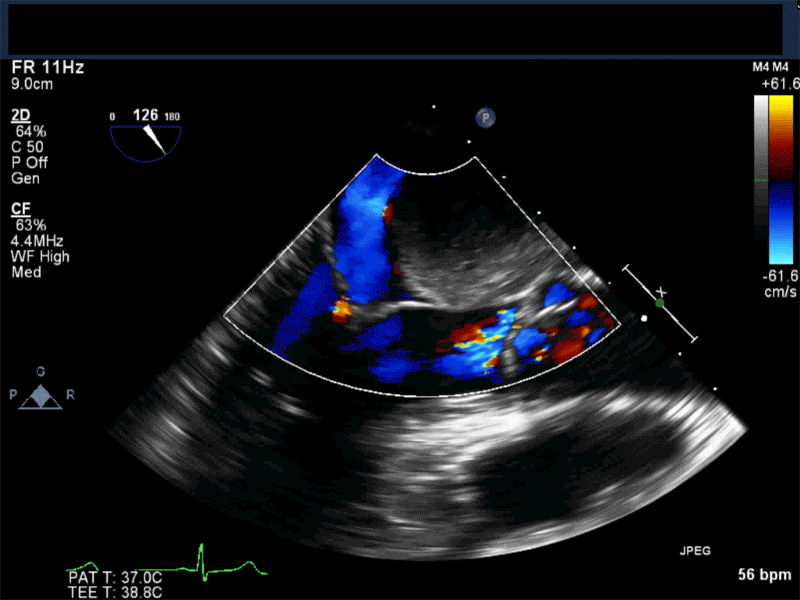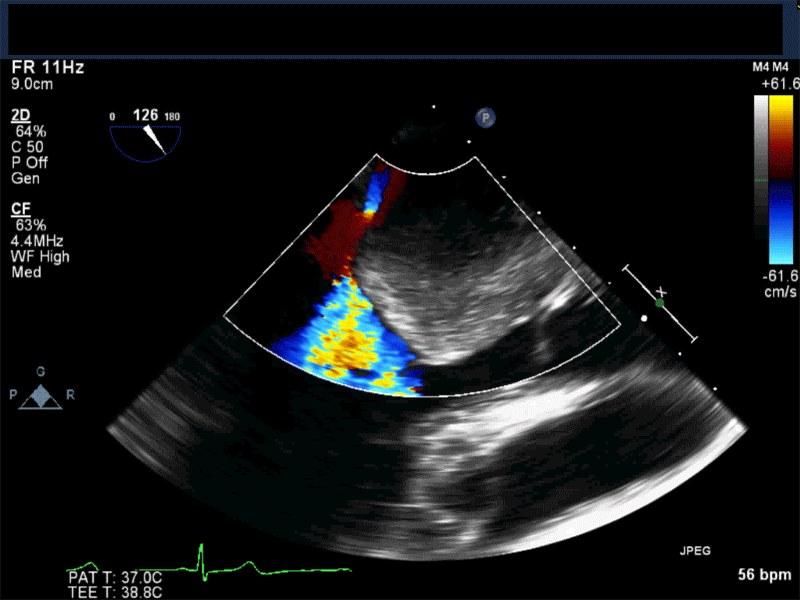
Video 3 Midesophageal 2-chamber view showing attachment of the myxoma not at the level of the fossa ovalis but rather in the vicinity of the left atrial appendage, possible ligament of Marshall.
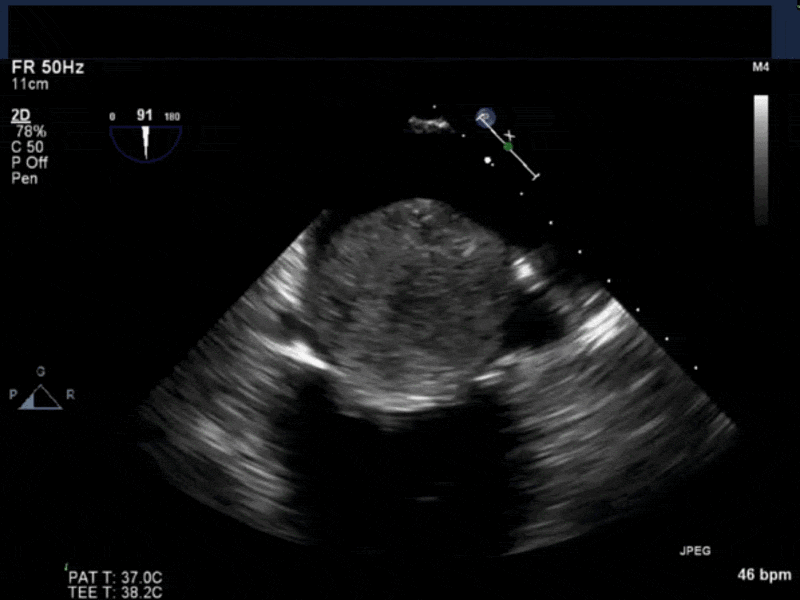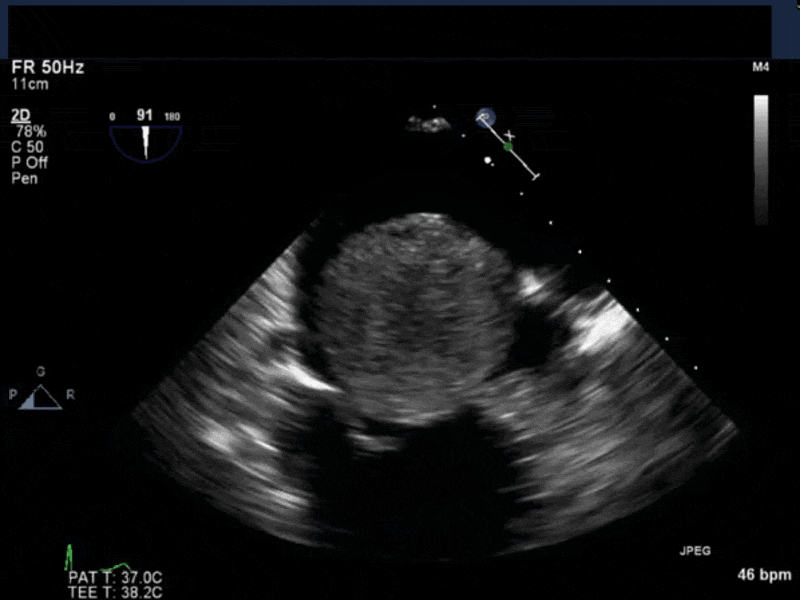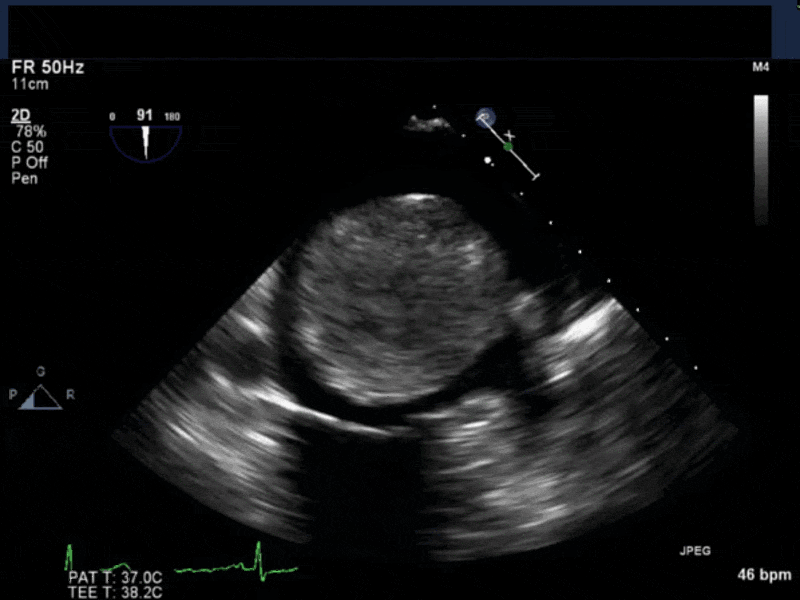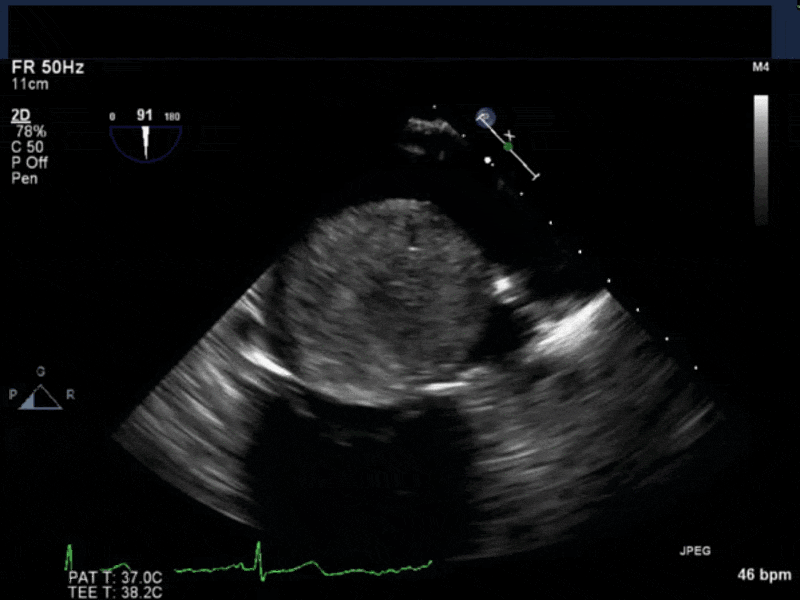
Video 4 3D-zoom view of the myxoma
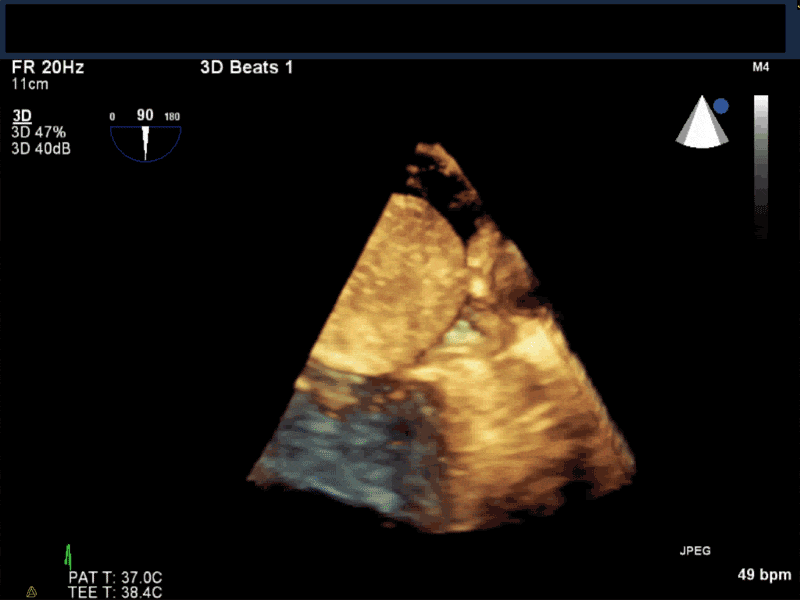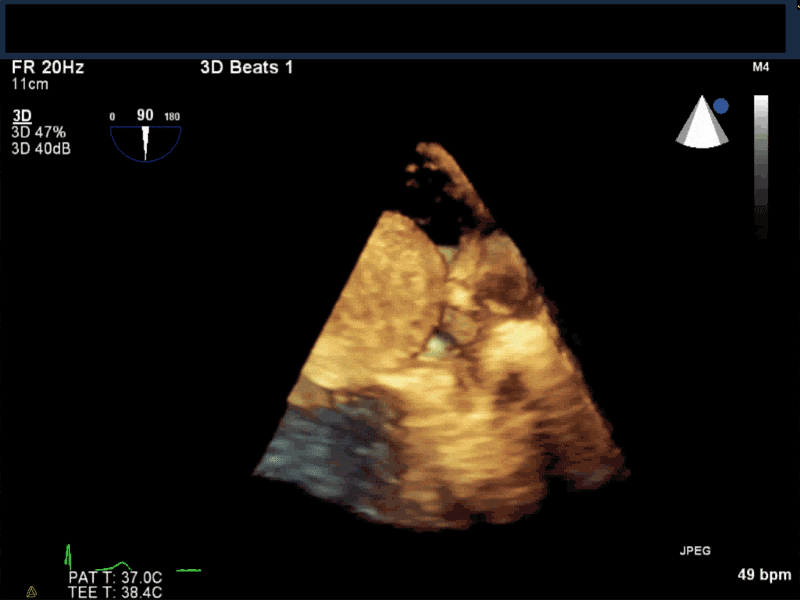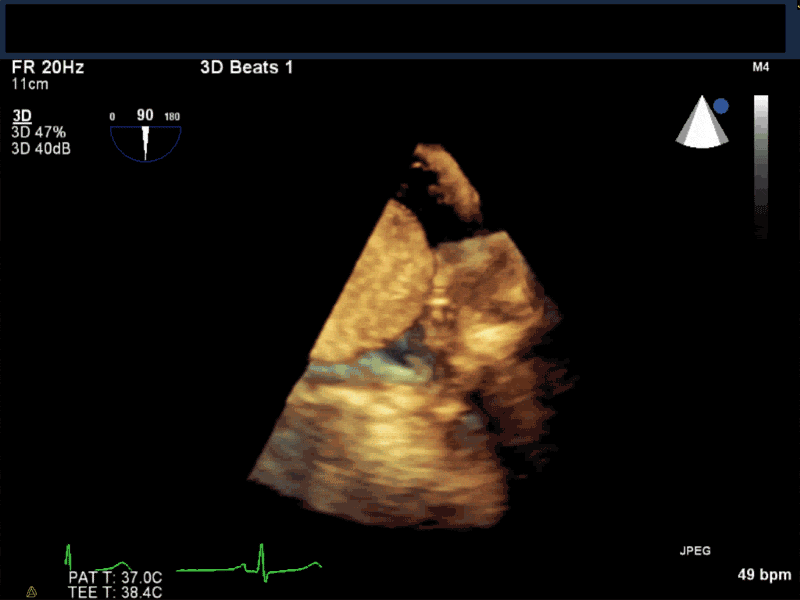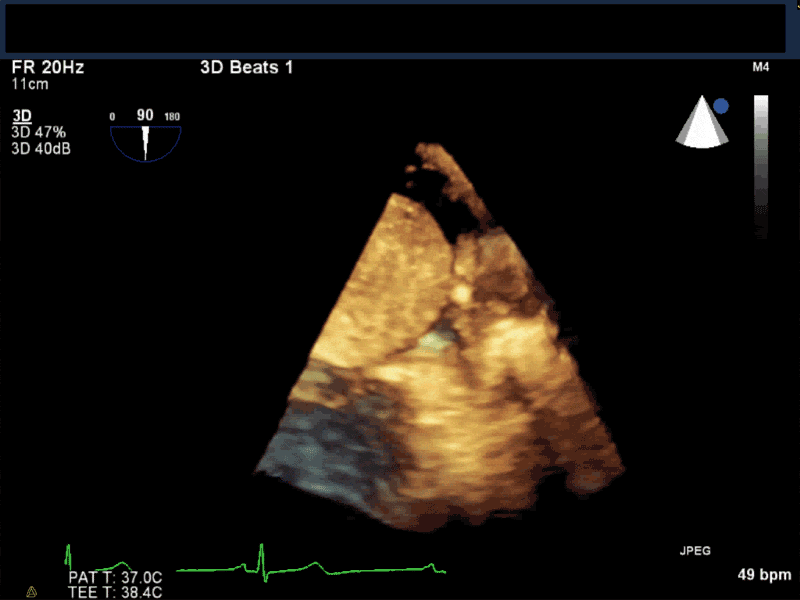
Video 5 En-face 3D view of the mitral valve showing large myxoma occupying almost entirely the mitral valve area.
Reference
Reynen, K Cardiac myxomas N Engl J Med 1995; 333:1610-1617
Most common congenital anomaly at birth but only 10-‐15% of defects in adults with congenital heart disease.
| Type | AKA | Description | % of all VSDs |
|---|---|---|---|
| Supracristal |
|
|
5 |
| Infracristal |
|
|
70 |
| Muscular | Located in central or apical trabecular portion of the septum | 20 | |
| Atrioventricular canal |
|
Posterior to membranous IVS between TV/MV
|
5 |
| VSD | Peak pressure (mmHg) | LA or LV dilatation | Pulmonary artery pressures |
|---|---|---|---|
| Restrictive | > 75 | No | Normal |
| Mod Restrictive | 25-75 | ↑ | ↑ |
| Nonrestrictive | < 25 | ↑↑ | ↑↑ |
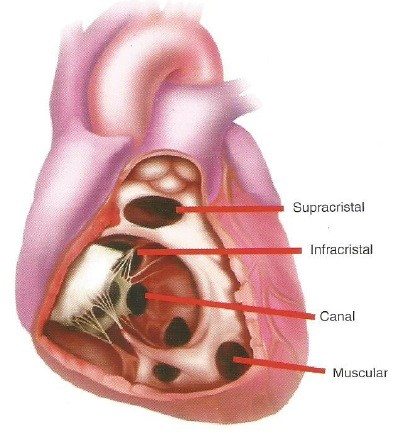
- 10% of perimembranous defects can undermine right aortic cusp causing herniation of cusp and AI
- Gerbode defect – rarely a perimembranous VSD can lead to communication between the LV outflow tract and right atrium
- Small membranous VSDs may close spontaneously during childhood by approximation of TV septal leflet across the defect
- Crista supraventricularis is synonymous with infundibular septum (muscle separating outflow tracts of L and R ventricles)
Echocardiographic Assessment
| Type | AKA | Echocardiographic View |
|---|---|---|
| Supracristal |
|
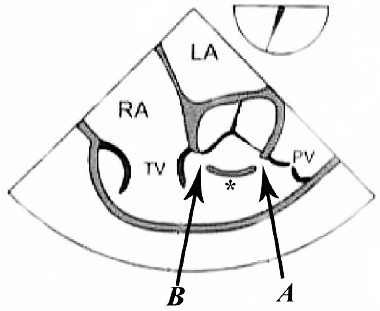
Midesophageal RV inflow outflow (figure 1-A) |
| Infracristal |
|
Midesophageal 5-chamber Midesophageal long axis Midesophageal RV inflow outflow (figure 1-B) |
| Muscular | Midesophageal 4-chamber Transgastric short-axis (basal, mid-papillary or apical) |
|
| Atrioventricular canal |
|
Midesophageal 4-chamber |
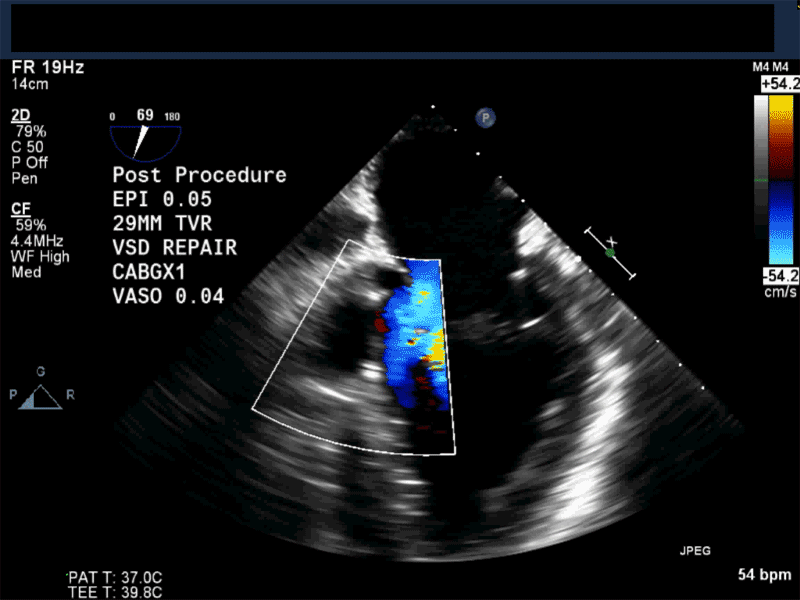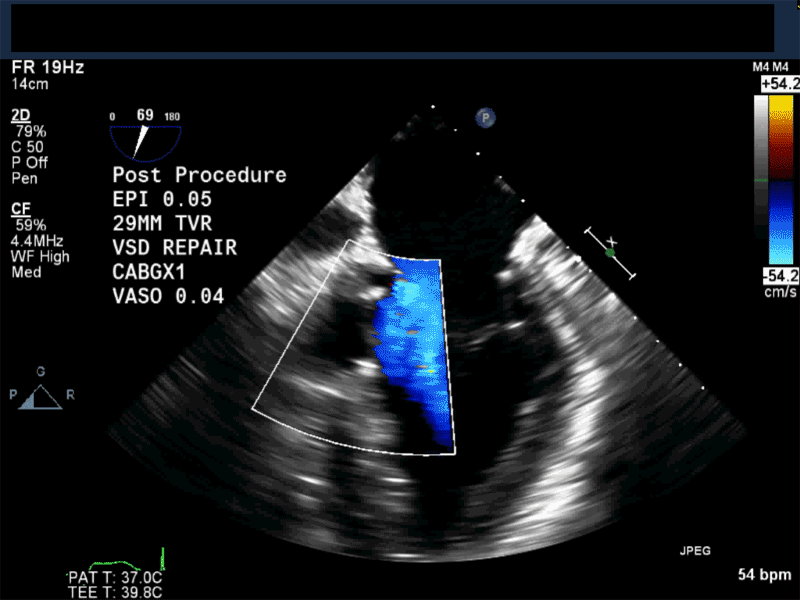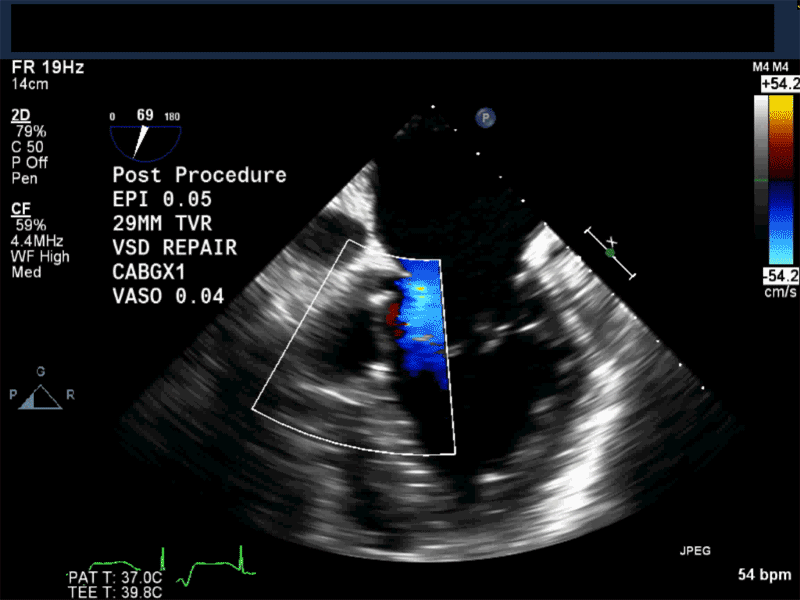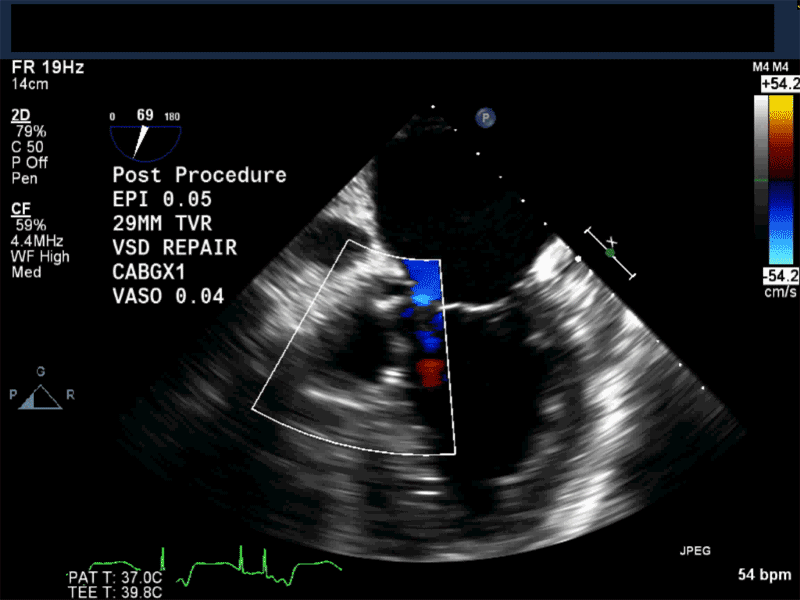
Video 1: Midesophageal RV inflowoutflow with color flow Doppler shows a small residual perimembranous VSD after patch repair.
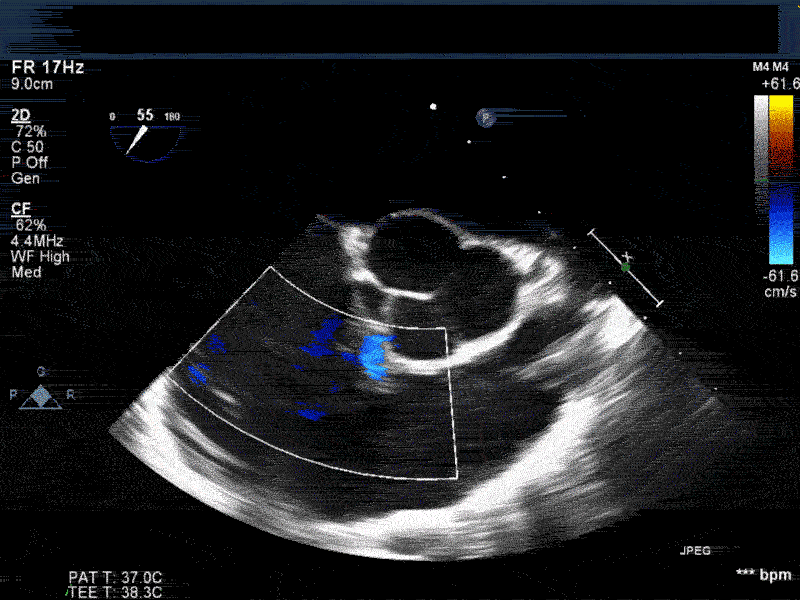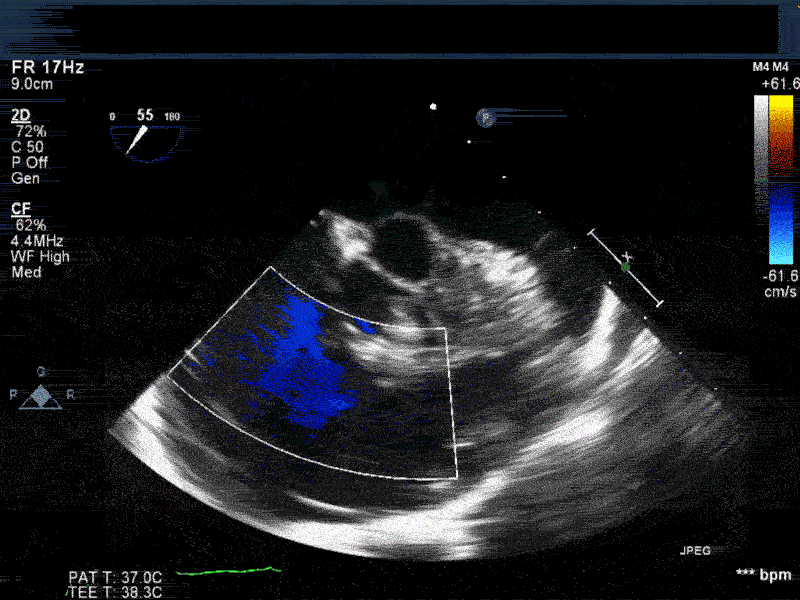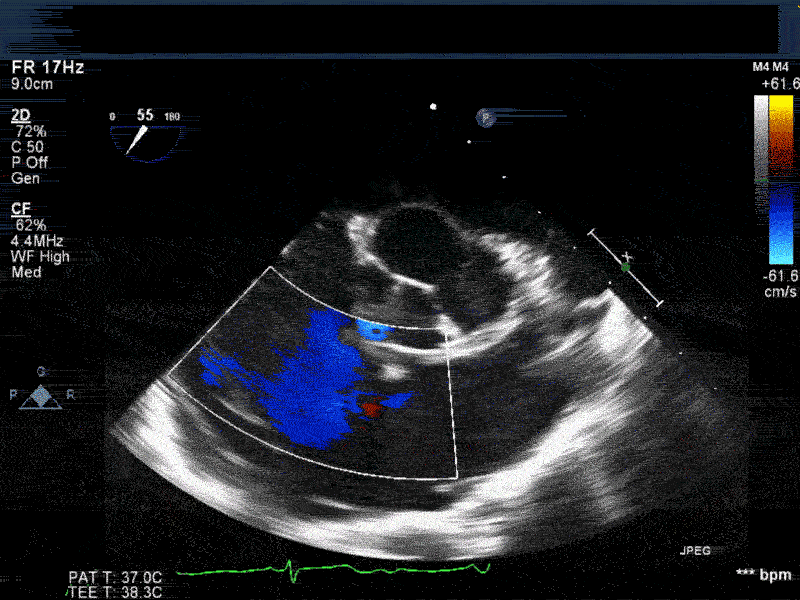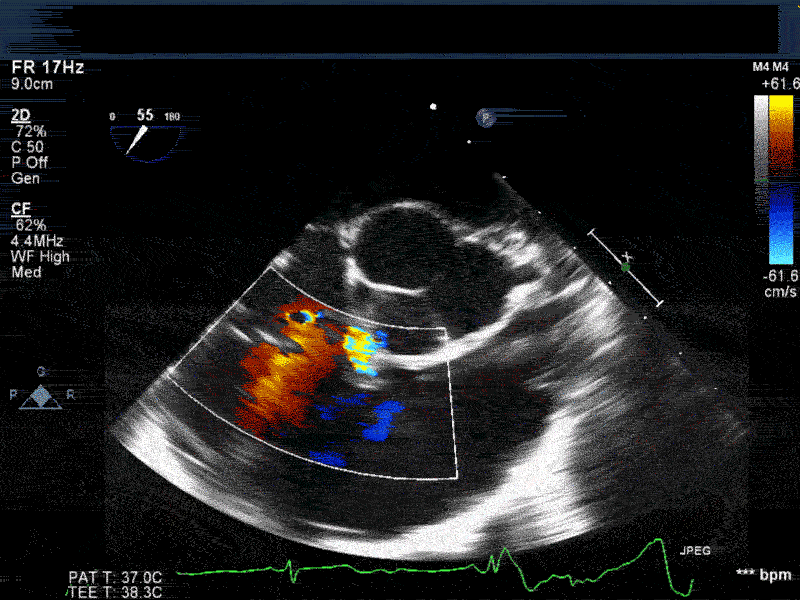
Video 2: Non-standard midesophageal mitral commissural view with the probe turned to the right shows blood flow through an ischemic muscular VSD opening under the tricuspid valve.
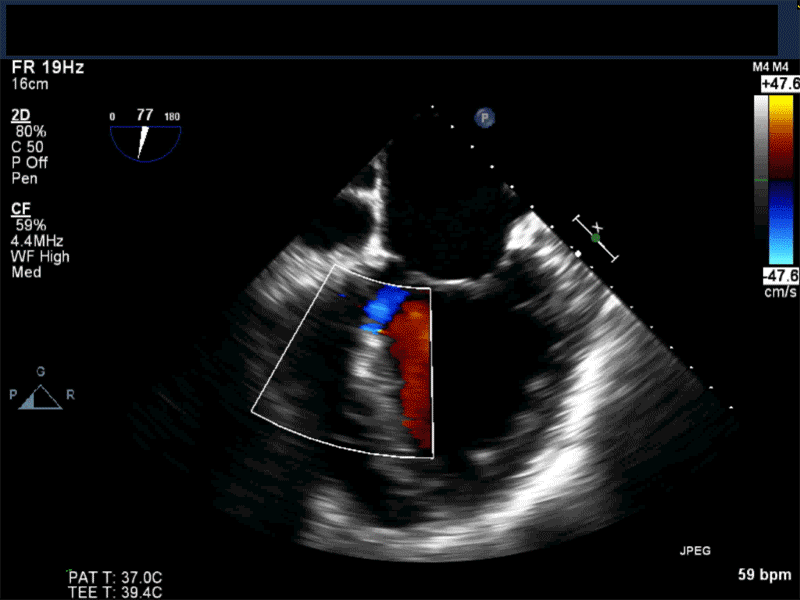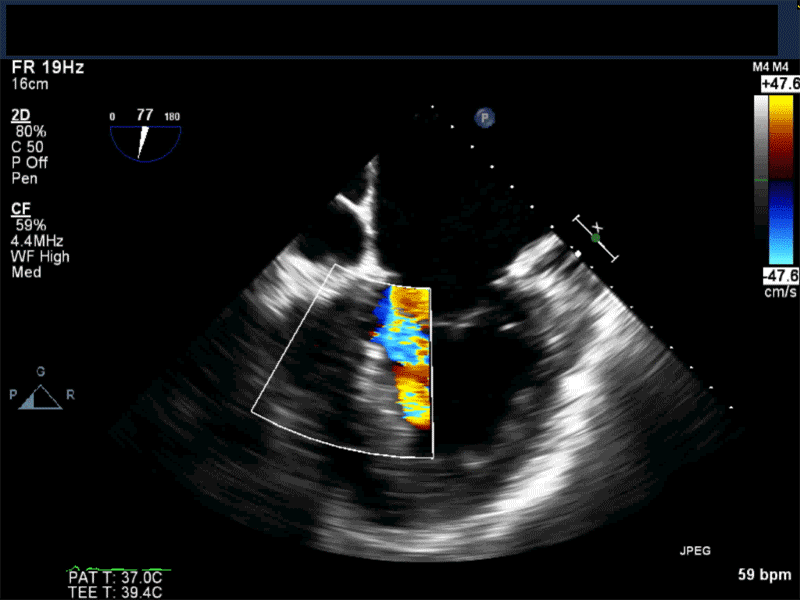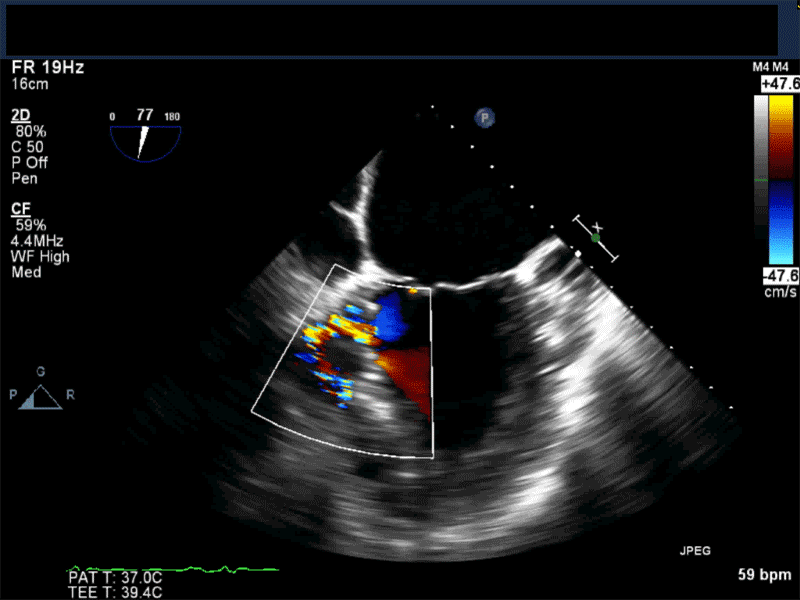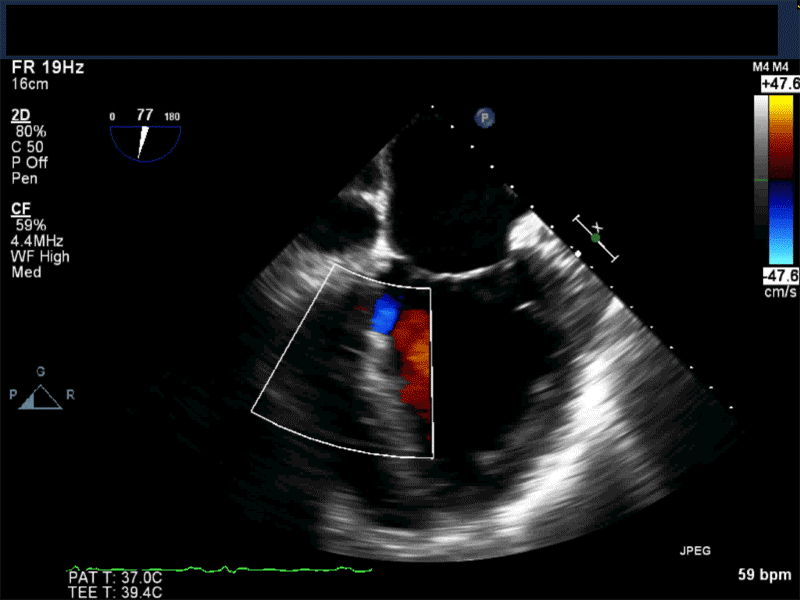
Video 3: Same patient as video 2. Transgastric short axis midpapillary view with color flow Doppler shows blood flow through a complex ischemic muscular VSD at the level of the inferoseptal wall. The VSD seems to open underneath the tricuspid valve at the level of the posteroseptal commissure.
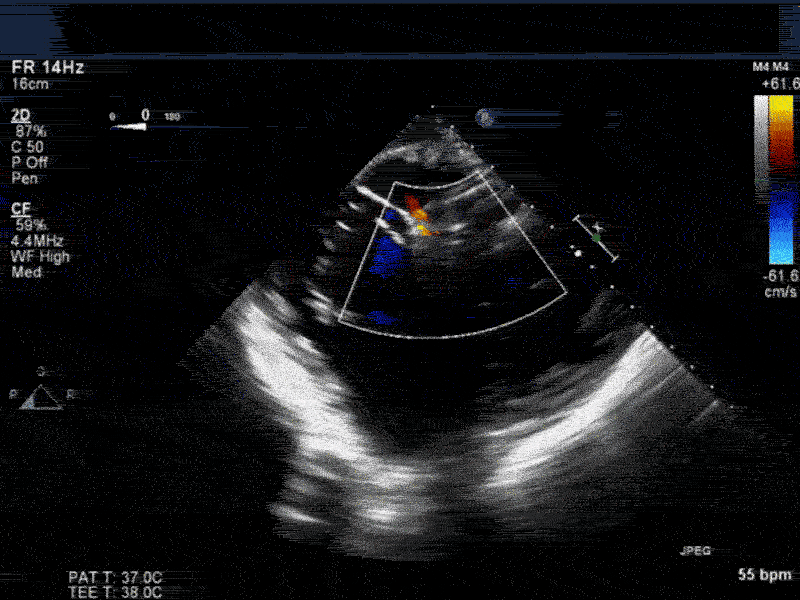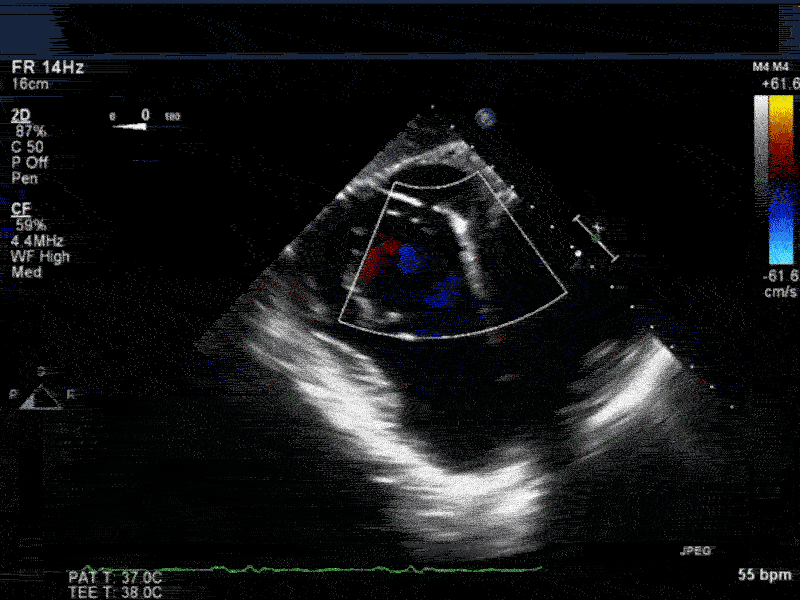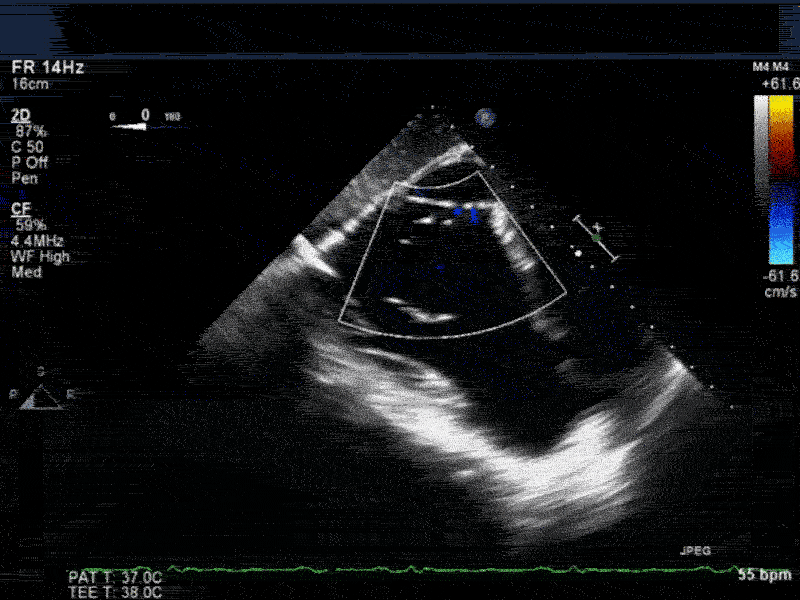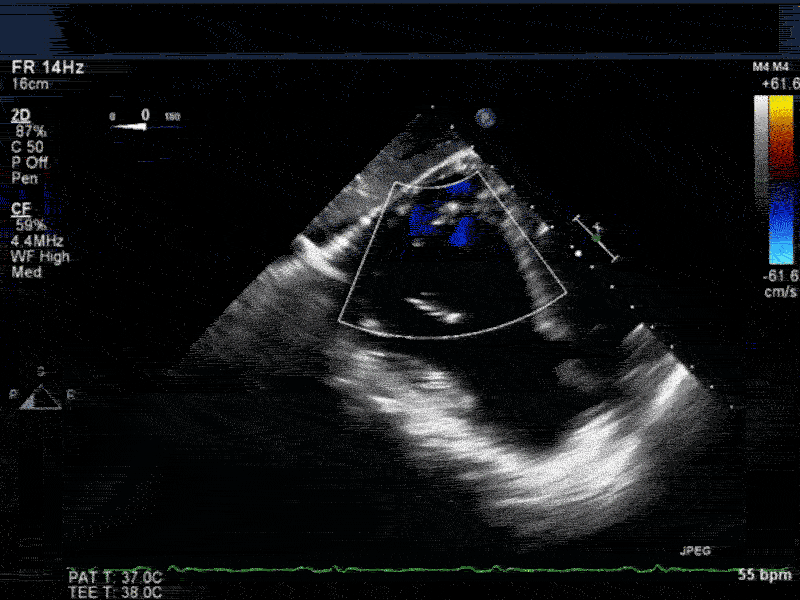
Video 4: Same patient as video 2 and 3. The patient underwent repair of the VSD with patch with approach through the right atrium and the tricuspid valve. The tricuspid valve was also replaced as the tricuspid valve had to be removed to facilitate access to VSD. Non-standard midesophageal mitral commisural view with the probe turned to the right shows the result of the patch repair.