
Mihai V. Podgoreanu, MD – 2012
2012 Final DIG Report | “Elucidating Adaptive Mechanisms of Perioperative Cardioprotection Following Ischemia-reperfusion in Hibernating Arctic Ground Squirrels”
The overall goal of this study is to understand how hibernating animals have developed natural defense mechanisms to withstand extremes of environment, and to ultimately apply this knowledge for organ protection in humans undergoing heart surgery. Three broad categories of regulatory mechanisms used by hibernating mammals to support long-term viability and organ preservation have relevance to perioperative cardioprotection. These include a controlled depression of the global metabolic rate, a shift in fuel utilization away from glucose towards fatty acids and ketones, and attenuation in the inflammatory response.
Findings
Our study began investigating the last two mechanisms, testing the joint hypothesis that changes in hibernation–specifically myocardial expression/activity of PPARα nuclear receptors following cardiac surgery–provide cardioprotection by increasing myocardial fatty acid oxidation (transactivation effects) and inhibiting NF-κB regulated pro-inflammatory responses (transrepression effects). The rationale for our study is that if endogenous activation of PPARα, or one of its target genes, in hibernators improves mitochondrial fatty acid utilization and avoids accumulation of incompletely oxidized lipid species while attenuating myocardial inflammation following cardiac surgery, then novel pharmacological cardioprotective approaches for perioperative metabolic optimization and reprogramming could be devised.
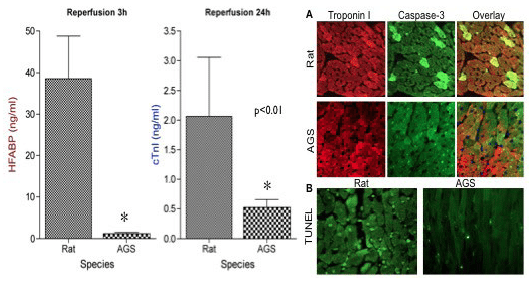
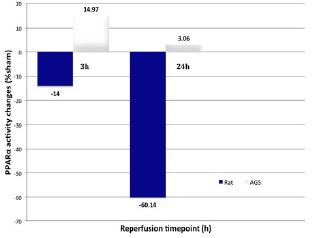
We conducted deep hypothermic/cardioplegic arrest (45 min, 18°C) followed by sham surgery, reperfusion for 3 hours, or reperfusion for 24 hours in adult hibernating arctic ground squirrels (AGS), summer active AGS, and rats. Compared to rats, AGS displayed robust tolerance of myocardial ischemia following DHCA as evidenced by reduced myonecrosis and apoptosis (fig. 1). This cardioprotective phenotype in AGS was associated with preservation of myocardial PPARα activity, and a metabolic phenotype consistent with the development of mitochondrial substrate flux “bottlenecks” in rats (accumulation of acylcarnitines and ceramides) (fig. 2).
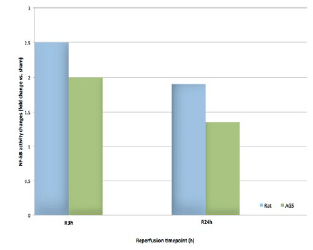
Furthermore, the hibernator cardioprotective phenotype was also associated with reduced myocardial NF-κB activity (fig. 3), reduced expression of downstream cytokines (TNFα, IL6), and neutrophil extravasation (MPO) following cardiac surgery.
These findings are novel because they challenge the current paradigm for metabolic optimization in human ischemic heart disease and heart failure that involves promoting glucose oxidation at the expense of fatty acid oxidation during reperfusion, by attempting to induce an “adaptive” substrate switch that is the exact opposite of that demonstrated by natural hibernators.
Funding
We have received funding from the Foundation of Anesthesia Education and Research and the Duke School of Medicine Voucher Program, and have recently submitted an NIH Transformative-R01 grant. Our results have been selected for podium presentations at various local, national, and international meetings, including the annual scientific sessions of the American Society of Anesthesiology and the American Heart Association.
Next Steps
Our future directions include mechanistic studies to follow-up on the relationship between deregulation of PPARα and several of its specific target genes using in vivo pharmacological modulation as well as gain and loss of function experiments in cardiomyocytes. Of note, one of these target genes (FGF21) has already been shown to induce torpor (a hibernation-like hypometabolic state) when injected into mice, but its role in cardioprotection remains unclear. We further aim to characterize changes in the abundance of the full spectrum of proteins expressed in the heart using proteomic analyses, as well as in the repertoire of inflammatory cells responsible for heart damage following cardiac surgery using flow cytometry analyses.