Lab Description

Pain is a multidimensional sensory and emotional experience that is important for our survival. Acute pain occurs in response to environmental stimuli and warns us of potential or actual tissue damage. In the event of actual tissue damage, pain serves to promote wound healing and repair. However, in some cases the pain outlasts the stimulus and becomes chronic. Once pain becomes chronic, it is no longer beneficial and, instead, becomes a disorder in and of itself.
Chronic pain is one of our nation’s most significant healthcare problems. Although our understanding of pain neurobiology has grown over the last several years, few new therapeutics have been developed. Thus, it is imperative that further research be conducted to better understand chronic pain; its causes, effects, and treatments.
There are three main objectives of our lab’s research in this area.
- To determine the factors that put some people, but not others, at risk for maladaptive chronic pain conditions. To achieve this objective, we study genetic, biological, and environmental factors associated with the initial onset of pain as well as its severity and duration. In addition, we are beginning to study factors associated with patient-centred outcomes, which may have the power to predict optimal management strategies for different individuals.
- To elucidate the mechanism(s) whereby genetic, biological, and environmental factors drive chronic pain. To achieve this objective, we integrate molecular genetics, animal models, and clinical epidemiologic measures in order to reveal pathogenic processes that are unique to as well as common across a particular condition or individual(s). This line of inquiry will provide novel targets for the development of individualized therapeutics for the management of chronic pain.
- To improve pharmacologic management of pain. To achieve this objective, we conduct pre-clinical studies to test the efficacy of new compounds and to optimize the efficacy of existing compounds in patient-relevant animal models.
Research
Persistent COMT-dependent Pain: Role of β-adrenergic Receptors
Work by our group and others suggests that complex pain conditions are due, in large part, to diminished activity of catechol-O-methyltransferase (COMT; an enzyme that metabolizes catecholamines), which results in elevated levels of cathecholamines and increased activity of β2/3-adrenergic receptors (β2/3ARs). β2- and β3ARs are uniquely positioned to promote peripheral and central sensitization by way of proinflammatory cytokines and nitric oxide. However, more work is necessary to understand the complex network of relationships that exists between peripheralm spinal, and central βARs and downstream signaling molecules and their relevance to persisitent pain.
The present studies employ a novel animal model or persistent pain produced by sustained COMT inhibition that closely mimics the persistent pain observed in FM, TMD, and related conditions to elucidate the role βArs play in driving prsistent pain. Specifically, we will 1) identify the subtype and location of βArs that contribute to persistent pain, 2) characterize the long-term consequences of sustained βAR activation on neurons and glia located in spinal and brain regions that relay pain information, 3) characterize the long-term consequences of sustained βAR activation on proinflammatory cytokines and NO, which represent validated markers of nociception, and 4) determine the ability of β2/3AR antagonistst to suppress the transmission of nociceptive information.
The outcome of these studies will provide new insights into mechanisms underlying maladaptive pain conditions, as well as contribute to the identification of previously unexploited targets (e.g., β2- and β3Ars) for development of effective therapies for patients with persistent pain conditions.
Molecular Profiling of Complex Persistent Pain Conditions to Identify Unique and Shared Pathways of Vulnerability
Ineffective treatment of common complex persistent pain conditions (CPPCs) such as fibromyalgia (FM), irritable bowel syndrome (IBS), vulvar vestibulitis syndrome (VVS), and episodic migraine (EM) constitutes a significant healthcare problem. These complex cinditions tend to co-occur and are characterized by a report of pain greater than would be expected based on a standard physical evaluation. The pathophysiology of CPPCs is largely unknown and conventional therapeutics possess limited efficacy and adverse side-effects. Thus, further research is imperative to better understand the mechanisms underlying FM, IBS, VVS, and EM. Identification of biological mediators that contribute to these conditions will lead to more accurate subdiagnoses and rational treatment strategies.
Emerging evidence indicates that polymorphic variations in genes coding for key regulators of pain-relevant pathways can produce a phenotype vulnerable to CPPCs. These genes and their corresponding proteins can be classified into one of three major clusters. Cluster 1 consists of those that influence the transmission of pain via peripheral afferents or central nervous system (CNS) pain processing systems (e.g., opioid, catecholamine, and ion channel pathways). Cluster 2 consists of those that mediate inflammatory responses to tissue injury and physiological stress (e.g., prostaglandin, glucocorticoid, and cytokine pathways). Cluster 3 consists of those that influence psychological state (e.g., catecholamine and serotonergic pathways). We hypothesize that common functional polymorphisms in these genes represent areas of genetic vulnerability, that when coupled with environmental triggers, will contribute to altered protein expression levels, enhances pain perception, and psychological dysfunction in individuals with CPPCs.
To test this hypothesis, the present studies will: 1) examine candidate gene polymorphisms in FM, IBS, VVS, and EM cases and pain free controls, using the Pain Research Panel that contains over 3,000 candidate single nucleotide polymorphisms (SNPs) in 360 genes, 2) measure expression of proteins encoded by candidate genes in FM, IBS, VVS, and cases and pain free controls by using a custom protein expression microarray, and 3) measure cell regulatory pathways in FM, IBS, VVS, and EM cases and pain free controls using FACTORIAL biosensor technology developed to assess the activity of multiple transcription factors simultaneously, generating a profile that represents a stable and sustained cell signaling signature. Secondary analyses will be conducted to explore the relationship between genotype, protein expression, regulatory processes, and clinical phenotype.
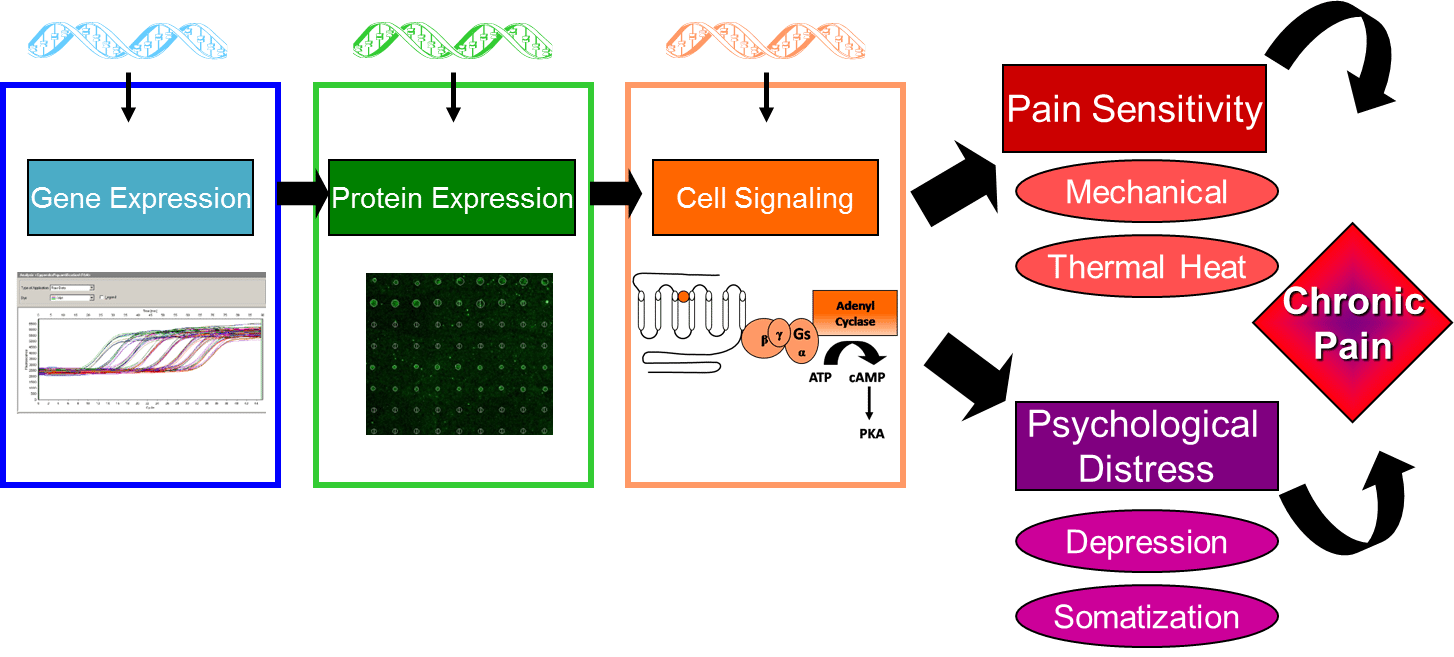
Elucidating the relationship between genotype, protein expression, regulatory processes, and clinical phenotype will provide new insights into mechanisms underlying maladaptive CPPCs. The outcome of these studies will contribute to the identification of unique genetic and molecular markers for the diagnosis of clinical pain conditions, as well as provide novel targets for the development of effective individualized therapeutics for CPPCs.
Characterizing the Role of a Newly Identified u1Opioid Receptor Isoform in Opioid-Induced Behavioral Phenotypes
Opioids are the most commonly prescribed analgesic for the treatment of clinical pain. However, significant individual variation exists in the degree of opioid analgesia and adverse side-effects (e.g., paradoxical hyperalgesia). This variation is influenced, in part, by allelic variations of the u1 opioid receptor (MOR-1). Recent work has revealed the existence of new exons within the human MOR-1 that contain polymorphisms associated with individual variability in pain sensitivity and MOR-1 agonist responses. Importantly, these polymorphisms are situated within a newly identified MOR-1 isoform that encodes a truncated version of the classical MOR-1 (MOR-1K). Stimulation of MOR-1K leads to excitatory signaling processes divergent from those of MOR-1. Preliminary studies in our lab reveal that MOR-1K transcript expression is enhances in mice exhibiting opioid hyperalgesia. These results suggest that MOR-1K may contribute to hyperalgesia versus analgesia. Additional studies to understand the functional characteristics of MOR-1K, and to determine its potential role in opioid hyperalgesia, are currently underway.
Extended Release of a Local Anesthetic for Continued Analgesia following Dental Extractions
The management of post-operative dental pain is imperative to eliminate needless patient suffering, improve health-related quality of life, and reduce health care costs resulting from additional clinical visists. Post-surgical dental pain is typically treated with orally administered analgesics that block either inflammatory mediators (e.g., NSAIDs, corticosteroids) or central mechanisms of pain perception (e.g., opioids). Although extremely beneficial for managing pain, these orally delivered drugs require continuous self-medication by the patient, and hold potential for adverse systemic effects, which in severe circumstances may result in neurological and respiratory dysfunction, nausea, circulatory depression, addiction, and sleep disruption.
Sustained delivery of a local anesthetic may slow down drug uptake into systemic circulation, thereby improving dental pain management by prolonging drug effectiveness and reducing toxicity. In this approach, a local anesthetic such as Bupivacaine is embedded within a moldable polymeric matrix; when residing within a tooth socket, a sustained dosing regime is enabled. This formulation, presented as a moldable gel or putty, will allow oral surgeons to customize the analgesic to the specific size and shape of a patient’s cavity. As the cavity heals, the anesthetic is continuously released and the matrix dissolved.
Awards and Recognitions
Congratulations to Dr. Andrea Nackley, who has been named a 2026 USASP Fellow—the highest honor of membership in the United States Association for the Study of Pain. Fellows will be formally recognized at the USASP Annual Scientific Meeting in Philadelphia, March 23–26, 2026. December 11, 2025
Congratulations to Ben Yacht, an undergraduate researcher in Dr. Andrea Nackley’s Translational Pain Research Laboratory, on being featured in the Duke University “Student Spotlight.” November 18, 2024
Sanjit Pamidi, a junior majoring in Neuroscience with a minor in Global Health and an undergraduate researcher with the Translational Pain Research Laboratory, was featured in the Student Spotlight on the Duke Neuroscience major’s website on August 12, 2024.
Congratulations to Nathaniel Hernandez whose poster abstract was selected from over 140 abstracts as a winner of the IASP Sex, Gender, Race and Pain Special Interest Group Poster Award. The committee was impressed by his clear commitment to research that advances the priorities of the SIG. July 10, 2024

Congratulations to Jen Ricano, whose abstract titled, “Activation of Beta-3 Adrenergic Receptors with the Selective Agonist Mirabegron Elicits Multi-Site Mechanical Hypersensitivity, Grimace, and Increased IL-6 Levels in Mice” wowed the judges and received first place in the Grad/Undergrad Research/CRNA/DNP category. May 7, 2024
In the February/March 2024 issue of Working@Duke magazine, Jen Ricano was quoted in the back cover feature titled "The Future: What are your predictions for Duke in the next 100 years?"
Richard Gao, an international student majoring in Neuroscience and minoring in Art History, was featured in the Department of Psychology & Neuroscience’s Student Spotlight. His research explores pain through both scientific and artistic lenses, focusing on Chronic Primary Pain Conditions and late 20th-century Chinese performance art. March 18, 2024
Congratulations to Nathaniel Hernandez on receiving this year’s 2024 US Association for the Study of Pain (USASP) Trainee/Young Investigator Travel Award in recognition of his contributions to pain research and his commitment to advancing the field. This award provides up to $1,000 to support Hernandez’s attendance at the USASP’s Annual Scientific Meeting in Seattle, scheduled for April 14-17, 2024.
Congratulations to Dr. Andrea Nackley, on receiving a two-year $1,846,202 R61 grant to develop novel Adrb3 antagonists for the treatment of chronic primary pain conditions as part of the NIH’s Helping to End Addiction Long-term (HEAL) Initiative. Read about her award.
Congratulations to Dr. Andrea Nackley, associate director of our Center for Translational Pain Medicine, on receiving a 2-year $50K grant from the National Vulvodynia Association for her 2023 project titled, “Inflammatory Mediators Associated with the Resolution of Pain.”
Congratulations to Dr. Andrea Nackley and Nathaniel Hernandez, BS (fellow), on receiving a $128,409 fellowship grant from the National Institutes of Health for their 2023 project aimed at advancing the understanding of the epigenetic basis for chronic primary pain conditions and elucidating new therapeutic targets. Read about their award.
Congratulations to Dr. Andrea Nackley, for her Novel Pain Therapeutics grant award from The University of North Carolina’s Eshelman Institute for Innovation entitled, “Peripherally-Targeted Adrb3 Antagonists for the Treatment of Pain.” Read about her award.
Congratulations to Nathaniel Hernandez, 2nd year graduate student, who was awarded the 2022 Diversity Equity and Inclusion Scholarship by the US Association for the Study of Pain.
Congratulations to Dr. Shin Hyung Kim, who was awarded first place in the Basic Science group during Duke Anesthesiology’s 28th Annual Academic Evening 2020 for his presentation on Elucidating the role of ectopic heat shock protein 90 (eHSP90) in inflammatory pain.
Congratulations to Viraj Patel, who was awarded a 2020 Summer Neuroscience Program Fellowship. This eight-week Duke program enables fellows to conduct full-time research to jump start their Graduation with Distinction senior thesis.
Congratulations to Scott Scarneo, recipient of the Keystone Symposium J7 scholarship for the February 2020 meeting Somatosensation: From Detection to Perception in Keystone, Colorado. The scholarship is generously supported by the National Institute of Neurological Disorders and Stroke, Grant #1R13NS113620-01.
Congratulations to Dr. Xin Zhang, recipient of the Keystone Symposium J8 scholarship for the February 2020 meeting Somatosensation: From Detection to Perception in Keystone, Colorado. The scholarship is generously supported by the National Institute of Neurological Disorders and Stroke, Grant #1R13NS113620-01.
R01 Grant Awarded to Dr. Andrea Nackley
Title: Defining the Role of Peripheral Adrb3 in Chronic Pain and Inflammation
Award Date: September 5, 2019
Congratulations to Andrea Nackley, PhD, on receiving $2,623,436 in funding from The NIH and the National Institute of Neurological Disorders and Stroke (NINDS).
Congratulations to Dr. Andrea Nackley, one of the 2019 DIG award recipients for her proposal to explore the molecular relationship between chronic pain and obesity. In collaboration with the Division of Metabolic and Weight Loss Surgery, the study aims to help identify molecular features of adipose tissue expansion that underlie comorbid chronic pain and obesity.
The Duke Clinical & Translational Science Institute (CTSI) has chosen to fund through the Duke/RTI Collaborative Translational Research Grants program, the application submitted by Dr. Andrea Nackley. The study title is “A New Peripherally-restricted Adrb3 Antagonist for the Treatment of Chronic Pain and Inflammation”
R01 Grant Awarded to Dr. Andrea Nackley
Title: Vestibulodynia: Understanding Pathophysiology and Determining Appropriate Treatments (Vestibulodynia: UPDATe)
Award Date: 9/11/2018
Congratulations to Andrea Nackley, PhD, on being awarded a 5-year, $3,846,094 NIH grant to conduct a multi-site clinical trial aimed at determining optimal therapies for patients suffering from a complex chronic vulvar pain condition.
26th Annual Duke Anesthesiology Academic Evening: May 29, 2018
1. Congratulations to Dr. Xin Zhang, whose abstract titled, “Activation of Peripheral β2 and β3ARs Leads to Increased Nociceptor Activity” wowed the judges and received first place in the Basic Sciences category.
2. Congratulations to Scott Scarneo, first place recipient of the best pre-doc, non-medical student category for his abstract titled, “Genetic and Pharmacological Validation of TAK1 Inhibition in Macrophages as a Therapeutic Strategy to Effectively Inhibit Inflammatory Pain.”
Congratulations to Julia Kozlowski, Duke senior undergraduate, on successfully presenting her scientific poster on April 24, 2018 as the concluding part for her Neuroscience Graduation with Distinction degree. Julia was mentored by Drs. Andrea Nackley and Xin Zhang, who also formed part of her thesis committee. Julia leaves our lab to become a medical student at the University of Michigan.

Dr. Xin Zhang received the Best Abstract Award for his poster titled “Activation of Peripheral β2 and β3ARs Leads to Increased Nociceptor Activity”at the 2018 Pain & Genetics SIG of the 37th annual APS meeting.
The Duke School of Medicine Multicultural Resource Center has nominated first-year medical student Zachary Smothers as a recipient of the “Charles D Watts Travel Award.” Zachary will attend the 37th Annual Scientific Meeting of the American Pain Society, to be held March 4 -6, 2018, in Anaheim, CA, where he will attend pertinent lectures and hold discussions with experts in the chronic pain field. This experience is aimed to increase his ability to better serve patients from diverse backgrounds.
Dr. Xin Zhang has been nominated by the 2018 Pain & Genetics Shared Interest Group (SIG) of APS for the ‘Best Abstract Award’. He will receive the award March 5, 2018, following a short presentation of his poster, “Activation of Peripheral β2 and β3ARs Leads to Increased Nociceptor Activity."
R03 Grant Awarded to Dr. Andrea Nackley
Title: Defining the Role of Adipocyte Adrb3 in Chronic Pain
Award Date: January 9, 2018
Congratulations to Andrea Nackley, PhD, on receiving $160,000 in funding to study peripheral mechanisms that drive chronic pain and work to develop peripherally-restricted therapies for patients with functional pain syndromes from The National Institute of Neurological Disorders and Stroke (NINDS).
2018 APS Young Investigator Travel Support Program awarded to Dr. Xin Zhang
Dr. Zhang will travel to, and present a poster at, the 37th Annual Scientific Meeting of the American Pain Society, held March 4 -6, 2018 in Anaheim, CA. APS gratefully acknowledges the National Center for Complementary and Integrative Health (NCCIH) for support of the Young Investigator Travel Award program. View Abstract
Dr. Andrea Nackley Appointed Co-Chair of Early Career Forum
The American Pain Society invited Dr. Nackley to become co-chair of the Early Career Forum. The appointment starts with co-planning the 2018 Scientific Summit with the current chair, continuing through 2019 when Dr. Nackley will be appointed the chair of the Early Career Forum.
Outstanding Poster Award awarded to Dr. Xin Zhang
Dr. Zhang received the award for his poster entitled, "Activation of Peripheral β2 and β3ARs Leads to Increased Nociceptor Activity" at the Translational Pain Research Symposium, Duke Kunshan University Kunshan, China, June 21-23, 2017.
Summary: Dr. Zhang showed that i) COMT inhibition leads to pain sensitivity, in line with increased ERK phosphorylation in DRG neurons and strengthened nociceptor activity in response to noxious stimuli, ii) COMT-dependent increases in pain sensitivity and nociceptor activity are driven by peripheral β2- and β3ARs, and iii) treatments targeted towards peripheral β2- and β3ARs and downstream effectors may prove useful in the management of functional pain syndromes.
2017 APS Young Investigator Travel Support Program awarded to Dr. Xin Zhang
Dr. Zhang will travel to, and present a poster at, the 36th Annual Scientific Meeting of the American Pain Society, held May 17-20, 2017 in Pittsburgh, Pennsylvania. APS gratefully acknowledges the National Center for Complementary and Integrative Health (NCCIH) for support of the Young Investigator Travel Award program.
Duke 2017 Summer Neuroscience Program Fellowship awarded to Katie Kanter
Title: Effects of MOR-1K Genetic Variation on Cellular Activity
Summary: Opioid induced hyperalgesia manifests as increased pain sensitivity due to acute or chronic opioid administration. A truncated variant in the mu opioid receptor, MOR-1K, has been linked to pain in human genetic studies, and shown to produce cellular excitation, resulting in hyperalgesia rather than analgesia.The Translational Pain Research Laboratory has identified a candidate single nucleotide polymorphism (SNP) within the enhancer box regulatory motif on MOR-1K exon 13 in CXB7/ByJ mice, that is predicted to contribute to the increased pain sensitivity observed in this variant compared to 129S6 mice. The proposed work will continue a previously unpublished study by the Translational Pain Research Laboratory, to elucidate alterations to MOR-1K receptor function related to this SNP using a cAMP assay, and ultimately examine changes to transcriptional regulation via a luciferase assay.
NIH/NIDCR Grant # R56DE025296-01 awarded to Dr. Andrea Nackley
Title: Proteins, MicroRNAs and Genes Associated with TMD and Overlapping Conditions
Awarded Date: September 21, 2016
Summary: The Institute of Medicine found that chronic pain affects 100 million Americans, causing extensive economic, social, and personal costs. For some the pain remains localized, while for others the pain spreads to affect multiple anatomic sites, suggesting a common underlying cause. In response to PA-14-244, we plan to use stored biospecimens and existing data from a clinical study of 1,460 adults to determine biological (proteins, microRNAs, and gene polymorphisms), psychosocial (stress, depression, anxiety), and clinical (general health and environmental exposures) factors that contribute to localized and overlapping pain conditions. Further, we will use bioinformatics methods to understand how these factors interact to influence pain, with the long-term goal to identify biomarkers for the diagnosis and treatment of chronic overlapping pain conditions.

Brittney Ciszek PhD, is the first-place recipient of the 24th Annual Duke Anesthesiology Academic Evening’s Excellence in the Pre-Doctoral Non-Medical Student category.
Bomi Oladosu has been selected to receive the GPSF Excellence in Mentoring Award 2016. The award will be presented at the 18th Annual Graduate School Student Recognition Ceremony, scheduled for April 14 at the George Watts Hill Alumni Center, University of North Carolina at Chapel Hill.
F31 from NIAMS/NIH awarded to Jane Hartung
Title: The Role of TNF-alpha and MAP Kinases in the Maintenance of COMT-Dependent Pain
Date: 09/01/2015 - 08/31/2017
Summary: This proposal utilizes a unique animal model that mimics the genetics and physiology of chronic musculoskeletal pain conditions along with behavioral pharmacologic, immunocytochemical, and live animal imaging techniques to test the hypothesis that tumor necrosis factor alpha (TNFα) and mitogen-activated protein kinases (MAPKs) maintain COMT-dependent pain at cellular and behavioral levels.
Lab Members
Publications
- Shudt C, Smith S, Bortsov A, Parr K, Gaynor S, Slade G, Zolnoun D, Nackley A. Vestibulodynia presentation is differentiated by the presence of additional chronic primary pain conditions. J Pain. 2025 May 22;33:105450. doi: 10.1016/j.jpain.2025.105450. Epub ahead of print. PMID: 40412493.
- Wang Y, Kim SH, Klein ME, Chen J, Gu E, Smith S, Bortsov A, Slade GD, Zhang X, Nackley AG. A mouse model of chronic primary pain that integrates clinically relevant genetic vulnerability, stress, and minor injury. Sci Transl Med. 2024 Apr 10;16(742):eadj0395. doi: 10.1126/scitranslmed.adj0395. Epub 2024 Apr 10. PMID: 38598615.
- Wang Y, Scarneo S, Kim SH, Zhang X, Chen J, Hughes P, Haystead T, and Nackley AG. Expression of ectopic heat shock protein 90 (eHsp90) in male and female primary afferent nociceptors regulates inflammatory pain. Pain 2021.
*Article will be highlighted on the Cover of the issue printed in May. - Zhang X, Kanter K, Chen J, Kim S, Wang Y, Adeyemi C, O’Buckley SC, Nackley AG. Low Catechol-O-Methyltransferase and Stress Potentiate Functional Pain and Depressive Behavior, Especially in Female Mice. Pain. 2020 Feb;161(2):446-458. PMID: 31972854
- Scarneo SA, Eibschutz LS, Bendele PJ, Yang KW, Totzke J, Hughes P, Fox DA, Haystead TAJ. Pharmacological Inhibition of TAK1, with the Selective Inhibitor Takinib, Alleviates Clinical Manifestation of Arthritis in CIA Mice. Arthritis Res Ther, 2019 Dec 17;21(1):292. PMID: 31847895
- Kim S, Zhang X, O’Buckley SC, Cooter M, Park JJ, Nackley AG. Acupuncture Treatment Reverses Chronic Pain and Neuroinflammation in a Mouse Model of Chronic Overlapping Pain Conditions. J Pain. 2018 Dec;19(12):1384.e1-1384.e14. doi: 10.1016/j.jpain.2018.05.013. Epub 2018 Jul 4. PMID: 29981376
- Zhang X, Hartung JE, Bortsov A, Kim S, O’Buckley SC, Kozlowski J, Nackley AG. Sustained Stimulation of β2- and β3-adrenergic Receptors Leads to Persistent Functional Pain and Neuroinflammation. Brain, Behavior, and Immunity. 2018 Oct;73:520-532. doi: 10.1016/j.bbi.2018.06.017. Epub 2018 Jun 20. PMID: 29935309
- Shepherd SD, O’Buckley SC, Harrington JM, Haines LG, Rothrock GD, Johnson LM, Nackley AG. A Moldable Sustained Release Bupivacaine Formulation for Tailored Treatment of Postoperative Dental Pain. Sci Rep. 2018 Aug 15;8(1):12172. PMID: 30111777
- Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018 Aug;129(2):343-366. PMID: 29462012
- Harmon JB, Sanders AE, Wilder RS, Essick GK, Slade GD, Hartung JE, Nackley AG. Circulating Omentin-1 and Chronic Painful Temporomandibular Disorders. J Oral Facial Pain Headache. 2016 Summer;30(3):203-209. PMID: 27472522
- Ciszek BP, O’Buckley SC, Nackley AG. Persistent Catechol-O-Methyltransferase-Dependent Pain Is Initiated by Peripheral β-Adrenergic Receptors. Anesthesiology. 2016 May;124(5):1122-35. PMID: 26950706
- Oladosua FA, Ciszek BP, O’Buckley SC, Nackley AG. Novel Intrathecal and Subcutaneous Catheter Delivery Systems in the Mouse. J Neurosci Methods. 2016 May 1;264:119-28. Epub 2016 Mar 11. PMID: 26976722
- Nackley A. Chronic Pain: Translating Discoveries Into Treatments. International Innovation. 2015 June;185:56-58.
- Oladosu FA, Conrad MS, O’Buckley SC, Rashid NU, Slade GD, Nackley AG. Mu Opioid Splice Variant MOR-1K Contributes to the Development of Opioid-Induced Hyperalgesia. PLoS One. 2015 Aug 13;10(8):e0135711. eCollection 2015. PMID: 26270813
- Oladosu FA, Maixner W, Nackley AG. Alternative Splicing of G Protein-Coupled Receptors: Relevance to Pain Management. Mayo Clin Proc. 2015 Aug;90(8):1135-51. Review. PMID: 26250730
- Hartung JE, Eskew O, Wong T, Tchivileva IE, Oladosu FA, O’Buckley SC, Nackley AG. Nuclear Factor-Kappa B Regulates Pain and COMT Expression in a Rodent Model of Inflammation. Brain Behav Immun. 2015 Nov;50:196-202. Epub 2015 Jul 15. PMID: 26187567
- Ciszek BP, Khan AA, Dang H, Slade GD, Smith S, Bair E, Maixner W, Zolnoun D, Nackley AG. MicroRNA Expression Profiles Differentiate Chronic Pain Condition Subtypes. Transl Res. 2015 Dec;166(6):706-720.e11. Epub 2015 Jun 24. PMID: 26166255
- Applebaum E, Nackley AG, Bair E, Maixner W, Khan AA. Genetic Variants in Cyclooxygenase-2 Contribute to Post-treatment Pain among Endodontic Patients. J Endod. 2015 Aug;41(8):1214-8. Epub 2015 Jun 14. PMID: 26081267
- Smith SB, Reenilä I, Männistö PT, Slade GD, Maixner W, Diatchenko L, Nackley AG. Epistasis Between Polymorphisms in COMT, ESR1, and GCH1 Influences COMT Enzyme Activity and Pain.Pain. 2014 Nov;155(11):2390-9. Epub 2014 Sep 16. PMID: 25218601
- Hartung JE, Ciszek BP, Nackley AG. β2- and β3-Adrenergic Receptors Drive COMT-Dependent Pain by Increasing Production of Nitric Oxide and Cytokines. Pain. 2014 Jul;155(7):1346-55. Epub 2014 Apr 13. PMID: 24727346
- Sanders AE, Maixner W, Nackley AG, Diatchenko L, By K, Miller VE, Slade GD. Excess Risk of Temporomandibular Disorder Associated with Cigarette Smoking in Young Adults. J Pain. 2012 Jan;13(1):21-31. Epub 2011 Oct 26. PMID: 22036516
- Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine Biomarkers and Chronic Pain: Association of Genes, Transcription, and Circulating Proteins with Temporomandibular Disorders and Widespread Palpation Tenderness. Pain. 2011 Dec;152(12):2802-12. Epub 2011 Oct 14. PMID: 22000099
- Gris P, Gauthier J, Cheng P, Gibson DG, Gris D, Laur O, Pierson J, Wentworth S, Nackley AG, Maixner W, Diatchenko L. A Novel Alternatively Spliced Isoform of the Mu-Opioid Receptor: Functional Antagonism. Mol Pain. 2010 Jun 2;6:33. PMID: 20525224
- Nackley AG, Diatchenko L. Assessing Potential Functionality of Catechol-O-Methyltransferase (COMT) Polymorphisms Associated with Pain Sensitivity and Temporomandibular Joint Disorders. Methods Mol Biol. 2010;617:375-93. PMID: 20336436
- Tchivileva IE, Tan KS, Gambarian M, Nackley AG, Medvedev AV, Romanov S, Flood PM, Maixner W, Makarov SS, Diatchenko L. Signaling Pathways Mediating Beta3-Adrenergic Receptor-Induced Production of Interleukin-6 in Adipocytes. Mol Immunol. 2009 Jul;46(11-12):2256-66. Epub 2009 May 23. PMID: 19477016
- Nackley AG, Shabalina SA, Lambert JE, Conrad MS, Gibson DG, Spiridonov AN, Satterfield SK, Diatchenko L. Low Enzymatic Activity Haplotypes of the Human Catechol-O-Methyltransferase Gene: Enrichment for Marker SNPs. PLoS One. 2009;4(4):e5237. Epub 2009 Apr 13. PMID: 19365560
- Tchivileva IE, Nackley AG, Qian L, Wentworth S, Conrad M, Diatchenko LB. Characterization of NF-kB-Mediated Inhibition of Catechol-O-Methyltransferase. Mol Pain. 2009 Mar 16;5:13. PMID: 19291302
- Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic Architecture of Human Pain Perception. Trends Genet. 2007 Dec;23(12):605-13. Epub 2007 Nov 26. Review. PMID: 18023497
- Diatchenko L, Slade GD, Nackley AG, Maixner W. Responses to Drs. Kim and Dionne Regarding Comments on Diatchenko, et al. Catechol-O-Methyltransferase Gene Polymorphisms are Associated with Multiple Pain-Evoking Stimuli. Pain 2006; 125: 216-24. Pain. 2007 Jun;129(3):366-370. No abstract available. PMID: 17851590
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 Adrenergic Receptor Activation Stimulates Pro-Inflammatory Cytokine Production in Macrophages via PKA- and NF-kappaB-Independent Mechanisms. Cell Signal. 2007 Feb;19(2):251-60. Epub 2006 Sep 20. PMID: 16996249
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human Catechol-O-Methyltransferase Haplotypes Modulate Protein Expression by Altering mRNA Secondary Structure. Science. 2006 Dec 22;314(5807):1930-3. PMID: 17185601
- Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W. Catechol-O-Methyltransferase Gene Polymorphisms are Associated with Multiple Pain-Evoking Stimuli. Pain. 2006 Dec 5;125(3):216-24. Epub 2006 Jul 11. PMID: 16837133
- Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic Pain Disorders–Pathways of Vulnerability. Pain. 2006 Aug;123(3):226-30. Epub 2006 Jun 13. Review. No abstract available. PMID: 16777329
- Hohmann AG, Neely MH, Piña J, Nackley AG. Neonatal Chronic Hind Paw Inflammation Alters Sensitization to Intradermal Capsaicin in Adult Rats: A Behavioral and Immunocytochemical Study. J Pain. 2005 Dec;6(12):798-808. PMID: 16326368
- Fecho K, Nackley AG, Wu Y, Maixner W. Basal and Carrageenan-Induced Pain Behavior in Sprague-Dawley, Lewis and Fischer Rats. Physiol Behav. 2005 Jun 2;85(2):177-86. PMID: 15924913
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic Basis for Individual Variations in Pain Perception and the Development of a Chronic Pain Condition. Hum Mol Genet. 2005 Jan 1;14(1):135-43. Epub 2004 Nov 10. PMID: 15537663
Events
Photos
















