Lab Description
Our lab studies the mechanisms underlying perioperative neurocognitive disorders, focusing on postoperative delirium, and how neuroimmune interactions ultimately impact cognitive outcomes during aging and neurodegeneration. Using an integrated interdisciplinary, collaborative, and translational approach, we are addressing the biological complexity of this common complication in the postoperative period by combining clinically relevant models with innovative bioengineering approaches and blood-based biomarkers. We aim to understand the role of neuroinflammation, with a particular focus on how brain barriers are affected following inflammation in order to develop safe strategies to resolve delirium and prevent subsequent cognitive decline following anesthesia and surgery.
Join our Team
The Terrando lab is well-funded via multiple NIA collaborative grants to study delirium pathogenesis and therapeutics. We are recruiting talented postdoctoral fellows, visiting scholars, graduate students, post-baccalaureate students, and undergraduate students. Interested candidates should email their CV with a brief cover letter describing research interests.
Major Research Interests
Neuroimmune Dysregulation in Postoperative Delirium
Our laboratory has a longstanding interest in how the immune system contributes to brain inflammation and cognitive outcomes after anesthesia and surgery. Current NIA-funded projects are focusing on:
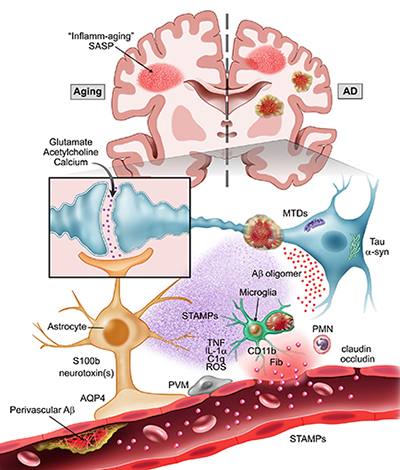
Aging: Every year, millions of older adults undergo surgery for medically necessary conditions and are at risk for developing postoperative neurocognitive disorders. After a routine operation, for example orthopedic surgery, many patients experience postoperative delirium (an acute confusional state characterized by inattention, diminished level of consciousness, thought disorganization, and a fluctuating course) that in some cases can presage the onset of neurodegenerative disorders such as Alzheimer’s disease and related dementias (AD/ADRD). We study the impact of aging on the immune system by focusing on key cellular components of the neurovascular unit (NVU), including endothelial cells, pericytes, and astrocytes, to understand how surgery-induced inflammation contributes to delirium. We leverage animal models with samples collected from clinical studies to validate and advance fluid biomarkers and characterize the underlying contributions for neuroimmune changes in delirium.
Delirium superimposed on dementia (DSD): Impaired cognition after common surgical procedures is a growing concern, especially among over 5 million people in the United States who suffer from dementia, including Alzheimer’s disease. After orthopedic surgery, acute changes in cognitive function, often referred to as postoperative delirium, occur in up to 89% of patients with preexisting dementia and are associated with poorer prognosis and even a two-fold greater risk for one-year mortality compared to patients without dementia or delirium. In our prior research, we have uncovered a key role for the disruption of the NVU in DSD. Current projects are exploring potential mechanisms for this pathology including: a) the role of fibrinogen in disrupting the blood-brain barrier (BBB) after surgery and activating microglial cells; b) the impact of blood-cerebrospinal fluid barrier and BBB opening in facilitating immune trafficking using aging and AD-like models; c) the contribution of amyloid-beta accumulation in DSD. We leverage collaborative and interdisciplinary team science to advance knowledge on these complex questions using integrated models and technologies. Key long-term collaborators on these projects include the Gelbard lab at the University of Rochester, New York, the Akassoglou lab at the Gladstone Institutes Neurological Disease, and the Maze lab at the University of California, San Francisco.
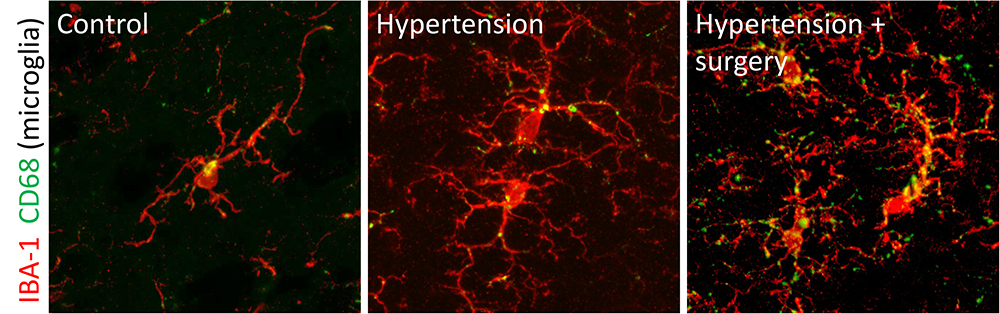
Hypertension and postoperative delirium: Millions of Americans suffer from cardiovascular disorders, including high blood pressure, and undergo routine surgical operations that pose high risks for neurologic complications, such as delirium and ADRD. The mechanisms whereby high blood pressure contributes to cognitive decline after surgery remain unknown. In collaboration with Dr. Ting Yang of Duke Medicine, we are testing the hypothesis that BBB dysfunction in hypertension primes the NVU by worsening border-associated immune activation and vascular dysfunction, thus exacerbating delirium-like behavior and ADRD after surgery and aging.
Therapeutics to Combat Postoperative Delirium
We have a long-standing interest in the role of “resolution of inflammation”, an active process where synthesis and release of anti-inflammatory molecules are required for balancing the overall immune response and healing. Our lab first reported on the effects of resolvins, lipid mediators generated by omega-3 essential fatty acids, in preventing neuroinflammation and memory deficits after surgery. Current studies are looking into the mechanisms of action of these molecules in the brain, especially focusing on Annexin-A1 and its neuroprotective properties on the BBB, immune cells trafficking, and DSD onset.
We also share an interest in bioelectronic medicine, and with Dr. Warren Grill of Duke BME we developed a novel percutaneous vagus nerve stimulator (US patent: US11590341B2) to help resolve neuroinflammation and improve cognitive outcomes. This approach has been successfully applied to postoperative pain following orthopedic surgery in studies with the Jordt lab and the Center for Translational Pain Medicine. Ongoing work is testing the efficacy of vagus nerve stimulation in the DSD model as a way to facilitate amyloid-beta clearance and reduce postoperative inflammation in AD-like mice. Results from the preclinical testing have also inspired the translation of this technology into a new clinical trial at Duke Health (study PI Dr. Leah Acker), which is evaluating delirium and other outcomes in older adults after surgery.
Ongoing projects are also testing how modulation of the IL-6 trans signaling is affected in delirium during aging and AD and how a mixed-lineage kinase (MLK) inhibitor developed by the Gelbard lab (URMC-099) may prevent aberrant neuroinflammation and NVU disruption in DSD.
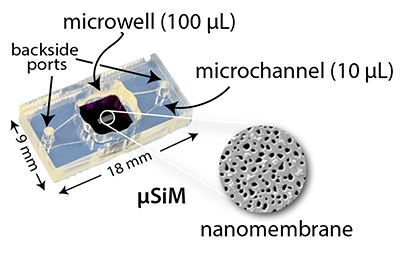
Our lab is part of the Translational Center for Barrier Microphysiological Systems (TraCe-bMPS), a consortium of industry and academia developing and applying chip-based models of human tissue for both discovery and regulatory drug development. We have multiple NIH-funded projects with the McGrath lab at the University of Rochester, New York, that apply microphysiological tools to deconstruct complex signals at the BBB by using the µSiM (microphysiological system featuring a Silicon Membrane) and modeling aspects of brain vulnerabilities, aging, protein aggregation, and hypertension using tissue chip platforms.
In the News
2025
June 5, 2025
NIH Grant Awarded to Advance Hypertension and Cognitive Decline Research
May 27, 2025
Neurologic Outcomes Research Group (NORG) Mini-Symposium
2024
August 14, 2024
Postdoctoral Fellow Awarded Alzheimer's Association Research Fellowship
April 11, 2024
Congratulations to Jamie Sokoloff on receiving the Dean’s Summer Research Fellowship Award
April 3, 2024
Inaugural Vascular and Neuroimmune Contributions to Brain Function Symposium
March 22, 2024
Dr. Terrando is a recipient of the 2024-25 Duke Faculty Advancement Seed Grant
2023
November 23, 2023
Dr. Terrando Nominated to the American Delirium Society Board of Directors
January 17, 2023
Dr. Terrando NIH Grant Renewed to Advance Delirium Research
2022
November 15, 2022
Dr. Terrando Awarded Nearly $5M to Study Neurocognitive Impacts of Surgery
June 29, 2022
Fish Oil Appears to Ease Post-Operative Delirium in Pre-Clinical Studies
October 11, 2022
Dr. Acker Awarded NIH Grant for HIPPIE Study
2021
August 17, 2021
Grant Awarded for Immunoprofiling Study
August 10, 2021
US Patent Awarded with Warren Grill, PhD and William Huffman (Duke BME) for Percutaneous Nerve Stimulation
2020
October 19, 2020
Drs. Yang, Velagapudi & Terrando review key mechanisms related to postoperative inflammation as driver of cognitive disorders.
May 5, 2020
NICCH-Funded Pain Center of Excellence
April 20, 2020
Study in Mice Suggests Post-Surgical Delirium Caused by Inflammation
March 30, 2020
Dr. Velagapudi Receives NIDUS Junior Investigator Pilot Award for his project aimed at developing novel therapies to treat delirium in patients with ongoing Parkinson’s disease pathology.
2019
October 29, 2019
Dr. Velagapudi Receives DIBS Germinator Award
May 28, 2019
Terrando and Grill Lab Member Awarded Wrenn Fellowship
April 16, 2019
Dr. Terrando Receives 2019 ISTAART New Investigator Award
April 2019
First book on Perioperative Neurocognitive Disorders Published
2018
October 11, 2018
Nerve Stimulation in Mice Suggests New Way to Reduce Delirium After Surgery
Lab moves to the the new MRSBIII building. Thank you all for the hard work!
July 18, 2018
William Huffman Presents on the Protective Effects of Vagus Nerve Stimulation
July 3, 2018
Research Grant: Bioelectronic Medicine Project Awarded with an R21
June 25, 2018
Research Grant: Effects of Omega-3 Fatty Acid on Postoperative Cognitive Dysfunction.
Thank you to the Terrando lab for the great talks.
2018
Neuroimmunology and Glia Group Retreat
2017
September 18, 2017
Research Grant: Dr. Terrando Receives Prestigious R01 Award
May 31, 2017
Postoperative cognitive dysfunction and Dr. Terrando’s research featured in Science
May 16, 2017
Chao Xiong “Runner Up” for the Post-Doc Basic Science Research Award at the 25th Duke Anesthesiology Academic Evening
2016
October 18, 2016
Pain signaling study published in PNAS and featured in the article
Research Grant: Role of TLR4 in Surgery-Induced Cognitive Decline
June 21, 2016
DIBS Research Incubator Award: Bioelectronic Medicine and Cholinergic Regulation of Postoperative Cognitive Dysfunction
Research Award: Neuroprotective Effects of URMC-099 in Postoperative Cognitive Dysfunction
2016 DIG Research Project
DIG Award: The Systemic Milieu and its Role in Postoperative Cognitive Dysfunction
Lab Members

Collaborators
Leah Acker, MD, PhD
Assistant Professor in Anesthesiology
Katerina Akassoglou, PhD
Professor and Senior Investigator
Gladstone Institutes
Harris Gelbard, MD, PhD
Professor
University of Rochester Medical Center
Sven Eric Jordt, PhD
Professor
Mervyn Maze, MB, CHB
Professor
University of California San Francisco
James McGrath, PhD
Professor
University of Rochester
William Wetsel, PhD
Professor
Director, Duke Behavioral Core
Ting Yang, MD, PhD
Assistant Professor
Selected Publications
- Miglietta AG, David-Bercholz J, Munji RN, Pulido RS, Soung AL, Terrando N, Daneman R, Yang T. Protocol for isolation of endothelial cells from adult mouse brain. STAR Protoc. 2025 May 21;6(2):103837. doi: 10.1016/j.xpro.2025.103837. Epub ahead of print. PMID: 40402746.
- Madhurakkat Perikamana S, Newman H, Vernon Shih Y, Duncan L, Rather HA, Li J, Velagapudi R, Terrando N, Varghese S. Depletion of senescent cells improves surgery-induced neuroinflammation in aged mice. PNAS Nexus. 2025 Apr 22;4(4):pgaf103. doi: 10.1093/pnasnexus/pgaf103. PMID: 40264849; PMCID: PMC12012716.
- Wu PY, Caceres AI, Chen J, Sokoloff J, Huang M, Baht GS, Nackley AG, Jordt SE, Terrando N. Vagus nerve stimulation rescues persistent pain following orthopedic surgery in adult mice. Pain. 2024 Aug 1;165(8):e80-e92. doi: 10.1097/j.pain.0000000000003181. Epub 2024 Feb 27. PMID: 38422485; PMCID: PMC11247455.
- Devinney MJ, Wong MK, Wright MC, Marcantonio ER, Terrando N, Browndyke JN, Whitson HE, Cohen HJ, Nackley AG, Klein ME, Ely EW, Mathew JP, Berger M; MADCO-PC and INTUIT Study Teams. Role of Blood-Brain Barrier Dysfunction in Delirium following Non-cardiac Surgery in Older Adults. Ann Neurol. 2023 Dec;94(6):1024-1035. doi: 10.1002/ana.26771. Epub 2023 Sep 25. PMID: 37615660; PMCID: PMC10841407.
- Vasunilashorn SM, Lunardi N, Newman JC, Crosby G, Acker L, Abel T, Bhatnagar S, Cunningham C, de Cabo R, Dugan L, Hippensteel JA, Ishizawa Y, Lahiri S, Marcantonio ER, Xie Z, Inouye SK, Terrando N, Eckenhoff RG; NIDUS Delirium Network. Preclinical and translational models for delirium: Recommendations for future research from the NIDUS delirium network. Alzheimers Dement. 2023 May;19(5):2150-2174. doi: 10.1002/alz.12941. Epub 2023 Feb 17. PMID: 36799408; PMCID: PMC10576242.
- Yang T, Velagapudi R, Kong C, Ko U, Kumar V, Brown P, Franklin NO, Zhang X, Caceres AI, Min H, Filiano AJ, Rodriguiz RM, Wetsel WC, Varghese S, Terrando N. Protective effects of omega-3 fatty acids in a blood-brain barrier-on-chip model and on postoperative delirium-like behaviour in mice. Br J Anaesth. 2023 Feb;130(2):e370-e380. doi: 10.1016/j.bja.2022.05.025. Epub 2022 Jun 29. PMID: 35778276; PMCID: PMC9997088.
- Zhang Z, Ma Q, Velagapudi R, Barclay WE, Rodriguiz RM, Wetsel WC, Yang T, Shinohara ML, Terrando N. Annexin-A1 Tripeptide Attenuates Surgery-Induced Neuroinflammation and Memory Deficits Through Regulation the NLRP3 Inflammasome. Front Immunol. 2022 May 6;13:856254. doi: 10.3389/fimmu.2022.856254. PMID: 35603196; PMCID: PMC9120413.
- Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020 Nov;21(11):1319-1326. doi: 10.1038/s41590-020-00812-1. Epub 2020 Oct 19. PMID: 33077953; PMCID: PMC7704062.
- Wang P, Velagapudi R, Kong C, Rodriguiz RM, Wetsel WC, Yang T, Berger M, Gelbard HA, Colton CA, Terrando N. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement. 2020 May;16(5):734-749. doi: 10.1002/alz.12064. Epub 2020 Apr 14. PMID: 32291962; PMCID: PMC7317948.
- Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. 2019 Jan-Feb;12(1):19-29. doi: 10.1016/j.brs.2018.10.005. Epub 2018 Oct 9. PMID: 30337243; PMCID: PMC6301148.
Click here for a complete listing of publications on PubMed (Terrando N)
Click here to view Dr. Niccolò Terrando Google Scholar Profile
Click here to view Dr. Niccolò Terrando’s Scholars@Duke Profile







