The center research targets physiological challenges faced during the exploration of environmental extremes from ocean depths to space exploration.
Diving related research includes:
- Research on human diving and altitude physiology including the mechanisms of immersion pulmonary edema, and, respiratory mechanics and gas exchange at non-standard pressures.
- Evidenced based diagnosis and treatment of decompression sickness and arterial gas embolization.
- Decompression sickness risk assessment for differing decompression algorithms.
- Non-clinical (laboratory) diagnosis of decompression illness.
The center faculty also conduct bench research, epidemiological research and clinical trials related to:
- Human diseases treated with hyperbaric oxygen including: necrotizing infections, osteoradionecrosis of the jaw, soft tissue radionecrosis and bisphosphonate associated osteonecrosis of the jaw.
- The molecular biology of metabolic gases (O2, CO NO).
- The clinical and molecular biology of wound healing
- Treatment of carbon monoxide poisoning.

Hypercapnia: Cognitive Effects and Monitoring
Jake Freiberger, MD, MPH, Bruce J. Derrick, MD, Tiffany Cobb, MSIII, Mike J Natoli, MS, Eric A Schinazi, Stephanie E. Martina, Michael Qin, PhD, Marlon Medford, MD, Judith H. Viola MD, Nicola Scafetta, PhD, Richard E. Moon, MD
NAVSEA Deep
Submergence Biomedical Contract #N0463A-12-C-0001
Goals: Reduce operational risk to the exercising diver
Navy need:
- Algorithm “equivalent narcotic depth” based on inspired breathing gas partial pressures
- PECO2 to PaCO2 correlation and effects are untested exercising at depth
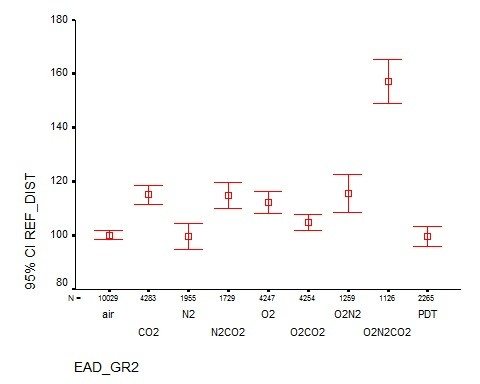
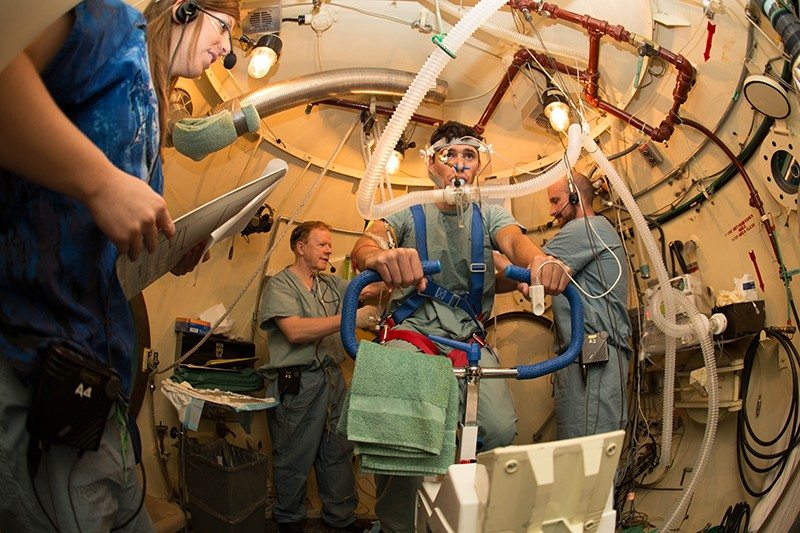
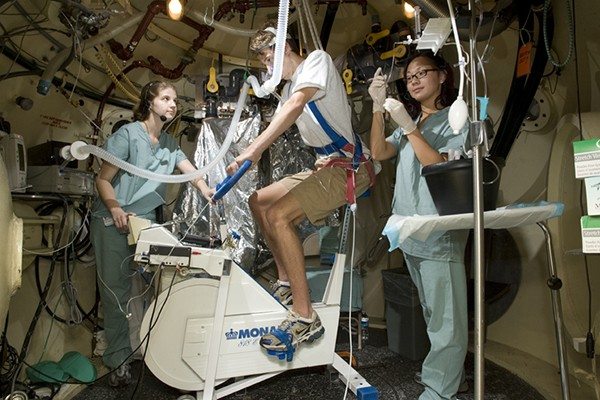
Ventilation perfusion mismatching during high altitude hypoxia impairs exercise ability. Can riociguat, a pharmacological inhibitor of hypoxic pulmonary vasoconstriction, alleviate this effect?
Impairment of exercise performance during altitude exposure is caused primarily by lower arterial oxygen content leading to reduced oxygen delivery to exercising muscles. Ultimately, hypoxemia results in hypoxic pulmonary vasoconstriction (HPV), causing pulmonary hypertension with further ventilation/perfusion (VA/Q) mismatch and PO2 decline. Therefore, if the HPV could be attenuated by pharmacologic blockade, then VA/Q matching, arterial PO2, and exercise performance should be improved. The nitric oxide (NO) pathway has been targeted in this context with sildenafil, but NO is limited at high altitude, which may explain the variable effectiveness of this approach. Riociguat is a novel soluble guanylate cyclase stimulator that does not require NO in its mechanism of action, so it may be effective in hypoxic and high altitude environments where NO availability is decreased. Subjects enrolled in this study will have radial arterial and pulmonary artery (PA) catheters placed prior to entering the hypobaric chamber. Subjects then perform incremental exercise tests on a bicycle ergometer at a simulated altitude of 15,000 feet before and after administration of riociguat. We compared radial and PA pressures, heart rate, ventilation, cardiac output, PaO2, SpO2, A-a difference, arterial oxygen delivery, and maximum work rate before and after administration of the drug. We hypothesize that riociguat will decrease pulmonary artery pressure and improve gas exchange and exercise performance at altitude.
Doxorubicin disrupts cardiac mitochondrial biogenesis, which promotes intrinsic apoptosis, while CO/HO promotes mitochondrial biogenesis and opposes apoptosis, forestalling fibrosis and cardiomyopathy.
(A) Representative gross and microscopic pathology. Left column: Cross-sections of mouse hearts fixed at a constant intracavitary pressure illustrate DOX-induced cardiomyopathy after 14 days. Normal mouse heart (control), CO control (CO), and DOX cross-sections are shown. CO administration, but not HH, preserves myocardial mass and LV wall thickness in DOX-treated mice. Middle column: Representative photomicrographs of LV sections of control, CO, DOX, and CO+DOX–treated mice stained with H&E (original magnification, ×250; scale bar = 10 μm). Normal myocardial morphology is shown (control). CO did not alter the myocardial morphology. DOX-damaged hearts showed extensive cytoplasmic vacuolization, myofibrillar loss, and cell death. Cardiomyocyte vacuolization and cell death were greatly reduced in animals that received CO, but swelling and vacuolization were still prominent after HH. Right column: Representative photomicrographs of mouse LV sections stained with Masson’s trichrome. Arrows indicate fibrosis of endomysium (light blue staining). Mice treated with CO showed less fibrosis after DOX. (B) Quantification of the interstitial fibrosis. Each bar represents mean ± SEM of 6 hearts. *P < 0.01 versus control; †P < 0.05 versus DOX. Note that CO protects, but HH does not.
Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007 Dec;117(12):3730-41.

Effect of Altitude Acclimatization on Lung NO and CO Concentration and Vascular Tone in Humans
(Drs. Bar-Yosef, Piantadosi, Moon). A joint study with members from the Department of Medicine examining the hypothesis that PO2-related changes in pulmonary and systemic vascular resistance in humans are mediated by changes in pulmonary NO and CO content. Analysis is in progress of data obtained from a prolonged exposure to 15,000 ft altitude in which volunteers are instrumented with arterial and pulmonary artery catheters.
Click on photo for information about the screening for IPE.

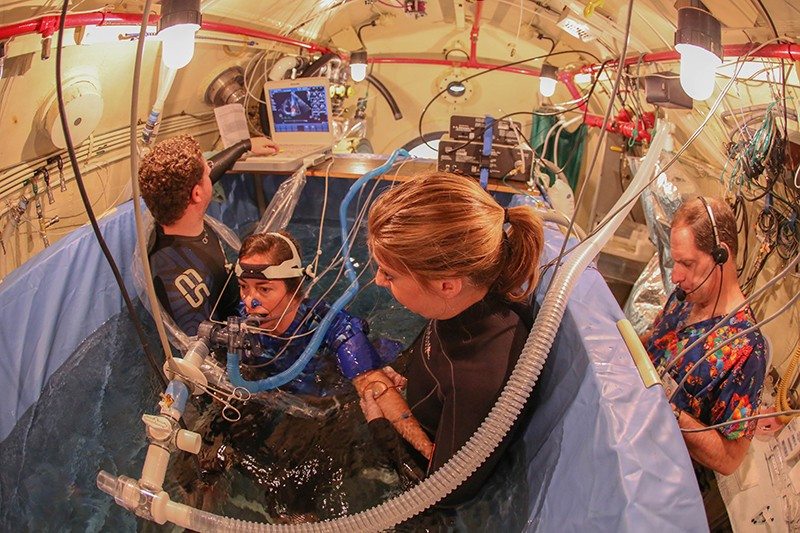
Immersion Pulmonary Edema Follow Up Study (click photo below)

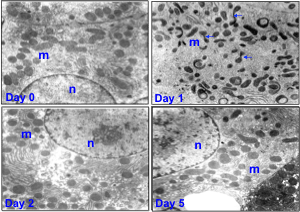
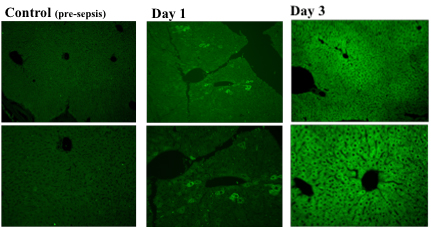
(Drs. Piantadosi, Welty-Wolf, Carraway). Animal models have been developed to study acute lung injury due to oxygen toxicity and sepsis. Methods of assessment include gas exchange (blood gases and multiple inert gas elimination), mechanics, lung morphometry, and measurement of pulmonary inflammatory responses and activation of the coagulation system. In addition, mechanisms or systemic organ injury, particularly those involving mitochondria, are being investigated.
Clinically, mitochondrial damage correlates with MODS and higher mortality. In sepsis mitochondria are damaged early and selectively, especially mtDNA. Damage mediated by ROS/RNS generated by the immune response promotes apoptosis. MtDNA transcription in vital organs is suppressed while nuclear genes are activated for mitochondrial proteins such as Tfam, Pol-γ.
Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med. 2003 Feb 15;167(4):570-9. Epub 2002 Dec 12.