
Complete the Anesthesiology Science Culture and Accountability Plan Attestation
Guiding Principles
The mission of the Department of Anesthesiology is to provide extraordinary care through a unique culture of innovation, education, research, and professional growth.
Research, being central to this mission, is highly integrated with education to provide avenues for medical students, residents, fellows, post‐doctoral associates, visiting research faculty, and regular faculty members to contribute novel information to the foundations and practice of anesthesiology and foster career development for scientists and physician‐scientists in the future.
All faculty, trainees, and staff involved in research at any level are expected to adhere to the highest of ethical and professional standards. This includes maintaining an environment that fosters mutual respect and teamwork, honesty, accountability, and open inquiry. Principal Investigators are ultimately responsible for the quality of research produced by their teams, and the highest priority is placed upon the integrity of the science produced within the department.
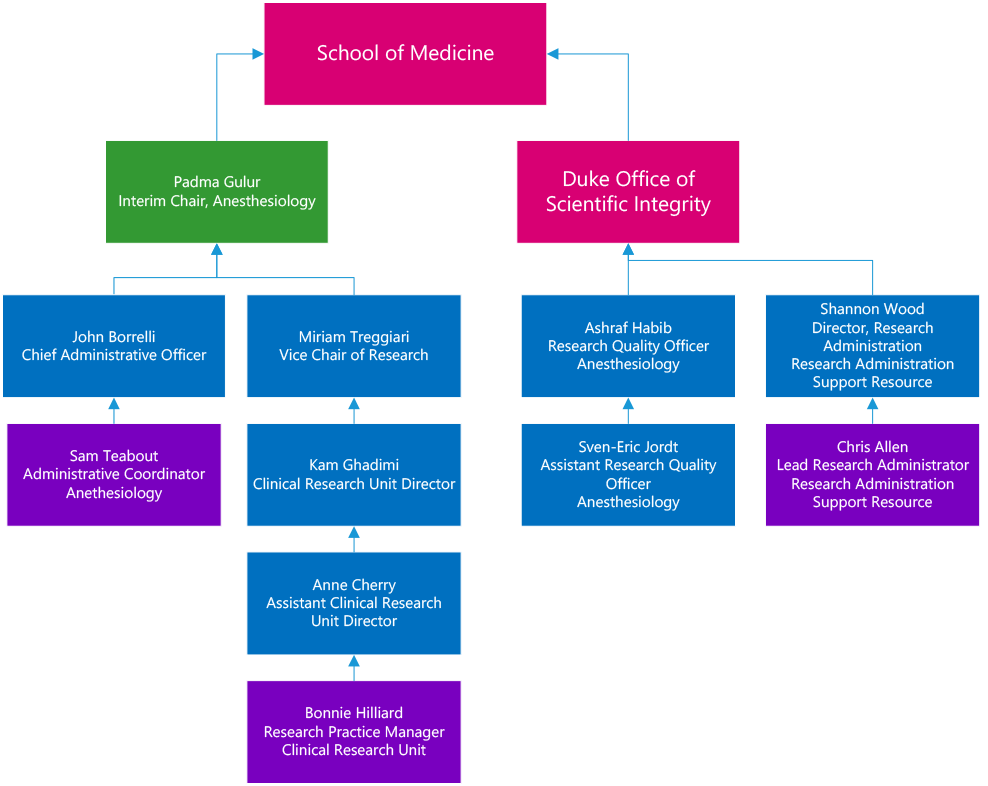
The Research Quality Team is comprised of:
- Ashraf Habib, MD, Research Quality Officer (RQO)
- Sven-Eric Jordt, PhD, Assistant Research Quality Officer (aRQO)
- Shannon Wood, Director Research Administration (RASR)
- Chris Allen, Grants and Contracts Manager, Lead Research Administrator (LRA)
- Bonnie Hilliard, RN BSN, Research Practice Manager
- Samantha Teabout, Administrative Coordinator
The RQO, aided by the Assistant RQO, has primary responsibility for addressing research integrity concerns, keeping research faculty and staff informed of relevant updates to institutional policies and procedures, assisting the Clinical Research Unit (CRU) with Clinical Quality Management Plans (CQMP) and data management plans (DMP), and working with the Advancing Scientific Integrity, Services and Training (ASIST) Team to track research related training compliance.
The team reports to the Vice Chair for Research, and directly to the Duke Office of Scientific Integrity (DOSI) and Office of Research Administration (ORA). Regular meetings are held among team members and between the team and DOSI/ORA as necessary.
Questions related to Scientific Culture and/or Accountability, or anything related to scientific integrity may be directed to any member of this team at any time. In addition, a report may be made via the department intranet directly to senior leadership, anonymously if so desired.
You can also find the feedback form by going to the top of the page, then click on the yellow Intranet button, then the confidential feedback button will on on the sidebar.
Research administration is overseen by the LRA in conjunction with the Anesthesiology Business Office and Anesthesiology Chief Administrator. The Anesthesiology Grants Team supports faculty members who are interested in submitting grant applications to federal, foundation, and industry organizations and institutions with the use of the Intent to Submit tool. Grant administrators and managers connect regularly with faculty and staff involved in funded research to address grant-related issues such as effort management, complying with both institutional and federal guidelines, and assistance with oversight responsibilities during the post award management and research project closeout process.
Education of Research Staff
- All faculty and staff engaged in research in any way are required to participate in the relevant Responsible Conduct of Research (RCR) Program. For new faculty and staff, information related to institutional policies and procedures, as well as expectations related to scientific integrity and accountability are included in the on-boarding process. Unit-level training is conducted periodically and tailored as much as possible to each specific unit.
- Changes to policies and procedures will be communicated to faculty and staff engaged in research regularly. This may occur at protocol or project meetings, or department-wide faculty meetings depending on the material to be presented.
- For Faculty and Staff pursuing grant funding, additional onboarding education and regular meetings with the grants team are conducted to ensure all parties are informed and accountable throughout the grant process.
- Faculty and staff engaged in research are encouraged to participate in educational opportunities outside the department related to professional development and scientific integrity (e.g. offerings through the Duke University School of Medicine Office for Faculty Development or the Career Development Seminar Series offered by the Department of Medicine).
Scientific Rigor & Reproducibility
Communication
- Principal Investigators within the department are expected to be responsible for the integrity of their research and the conduct of those involved in their investigations. While trust among team members is important, a culture of honesty, accountability, and constructive criticism is also critical. PIs are expected to be role models of this behavior, and should support each other in this pursuit.
- Biannual meetings are held with research-track trainees and junior faculty, their research mentors and senior leadership to evaluate the quality and status of research accomplished to date. This formal mentoring event incorporates all aspects of the conduct of research, including integrity and accountability.
Research Methods, Study Design
- Within each of the Research Centers (Center for Hyperbaric Medicine & Environmental Physiology, Center for Perioperative Organ Protection, Center for Translational Pain Medicine, and Critical Care & Perioperative Epidemiologic Research) regular meetings, ideally weekly, are held at which investigators at all levels present and discuss projects currently in progress. The aim of these meeting is to garner critical feedback that addresses the progress of ongoing research, analytical plans and methods, as well as development of new forward-looking research ideas.
- Research study design should ensure reproducibility. This includes establishing and adhering to Data Management Plans (see below) and creating a repository of methods protocols to be documented in the electronic lab notebook records and made available to all lab members, as described below. Randomization and blinding of samples are essential to reduce selection bias and subsequent biases. Sample sizes are to be determined by power analysis. These measures allow reliable statistical testing and are critical, particularly to the interpretation of preclinical proof-of-concept studies. It is recommended that a second researcher or team in the lab be tasked to replicate pivotal experiments. As per NIH mandate, validation of resources such as mouse strains, cell lines, chemicals, and other reagents is essential and should be documented to ensure reproducibility.
Data Management, Storage, Provenance
- Duke University’s data retention policy requires that all data are retained for at least six years. For details about this policy and all data handling and retention procedures, please consult: https://policies.provost.duke.edu/docs/chapter-5-research-data
- All basic and translational research laboratories in the Department of Anesthesiology are expected to utilize an electronic research notebook (ERN) for daily documentation of activities and results. The current application offered by the University is LabArchives and is available free of charge to departmental investigators. Without exception, all data are to be documented electronically. Clinical researchers are required to follow all sponsor-mandated policies for documentation of activities and data. The standard database for recording clinical research data is RedCap. For the case that clinical samples are processed in research labs, investigators should consider using LabArchives to record activities and data related to methods development and daily lab activities.
- All laboratories are expected to create a Data Management Plan (DMP) describing how they will collect, process, manage, store, and potentially share data. These DMPs should be reviewed and updated annually, and submitted to the Vice Chair for Research for archiving. This plan should address issues of roles & responsibilities of team members, training, research methods and data flow, data storage, organizational workflow, and data sharing. It is also understood that all team members are assigned duties based on their expertise and ability, and all understand their responsibilities as good data stewards.
- A DMP should be created for each clinical trial conducted within the department, along with a Statistical Analysis Plan, and if relevant, a Data Sharing Plan. These should be archived and made available for review as a ‘locked’ document upon request.
- Assistance with preparing a DMP is available: e.g. the Duke Management Guidance document.
- All raw, processed and analyzed data used in publications need to be clearly identifiable and traceable in ERNs or RedCap, and external repository records identified where these data are deposited to fulfill sponsor and publisher requirements.
- All research data collected by research personnel or other authorized individuals from electronic sources, patients or other IRB-approved collection methods are stored in secure locations with access strictly restricted to study team members. For activities deemed exempt by the IRB, data are either stored in Duke’s Protected Analytics Computing Environment (PACE) or in designated, secure storage on department servers only accessible by departmental statisticians and IT personnel.
- When a lab member is leaving a lab, the Principal Investigator and lab member are required to follow the procedures outlined in the the Data Offboarding Checklists provided by the School of Medicine in myRESEARCHpath. Two templates are available, one for non-human subject data, the other for human subject data. Duke has a data retention policy of 6 years.
Voicing Concerns
- Members of the Research Quality Team, especially the RQO and aRQO are available at any time to discuss any and all concerns from members of the department research community. Raising a concern about research integrity is not equivalent to accusing someone of misconduct; only by raising and routinely responding to concerns related to scientific integrity and establishing processes and a culture that encourage and supports ethical behavior can the highest standards of conduct become, in fact, standard.
- In order to manage issues that arise in which a party may feel disengaged, perceive conflict, or have concerns about research integrity, we have developed a policy regarding departmental reporting of research‐related grievances and concerns.
- In addition to contacting any member of the Research Quality Team, anonymous reporting of issues or concerns is possible using a link on the department intranet, created to support concerned individuals who wish to remain unidentified. Once a report is electronically submitted, the Vice Chair for Research and the Department Chair are automatically and immediately notified. Under their direction, he Research Quality Team is responsible for initial screening of these reports. Reports are addressed on a rolling basis and appropriate action taken. DOSI is engaged as indicated.
- Other resources in the School of Medicine and the University are available for reference, assistance, and/or reporting of events, concerns or conflicts.
| Research Integrity | Duke Office of Scientific Integrity Anonymous Duke Integrity Hotline: 1-800-826-8109 Duke’s Misconduct Review Officer: 919-668-5115 |
| Financial Conflicts of Interest | Duke Office of Scientific Integrity-Conflict of Interest (DOSI-COI) |
| Human Subjects: DUHS | Institutional Review Board |
| Animal Subjects | Duke Animal Care & Use Program |
| Workplace Environment | Occupational & Environmental Safety Office Office of Institutional Equity |
| Ombudspersons: School of Medicine Trainees | Student & Postdoctoral: Jean Spaulding, 919-668-3326 or ombudsman@mc.duke.edu |
| Ombudspersons: School of Medicine Faculty | Laura Svetkey, 919-681-6386 or laura.svetkey@duke.edu |
Page Updated: 12/8/2025